SWIPE TABLE TO VIEW MORE
SKYRIZI SAFETY PROFILE
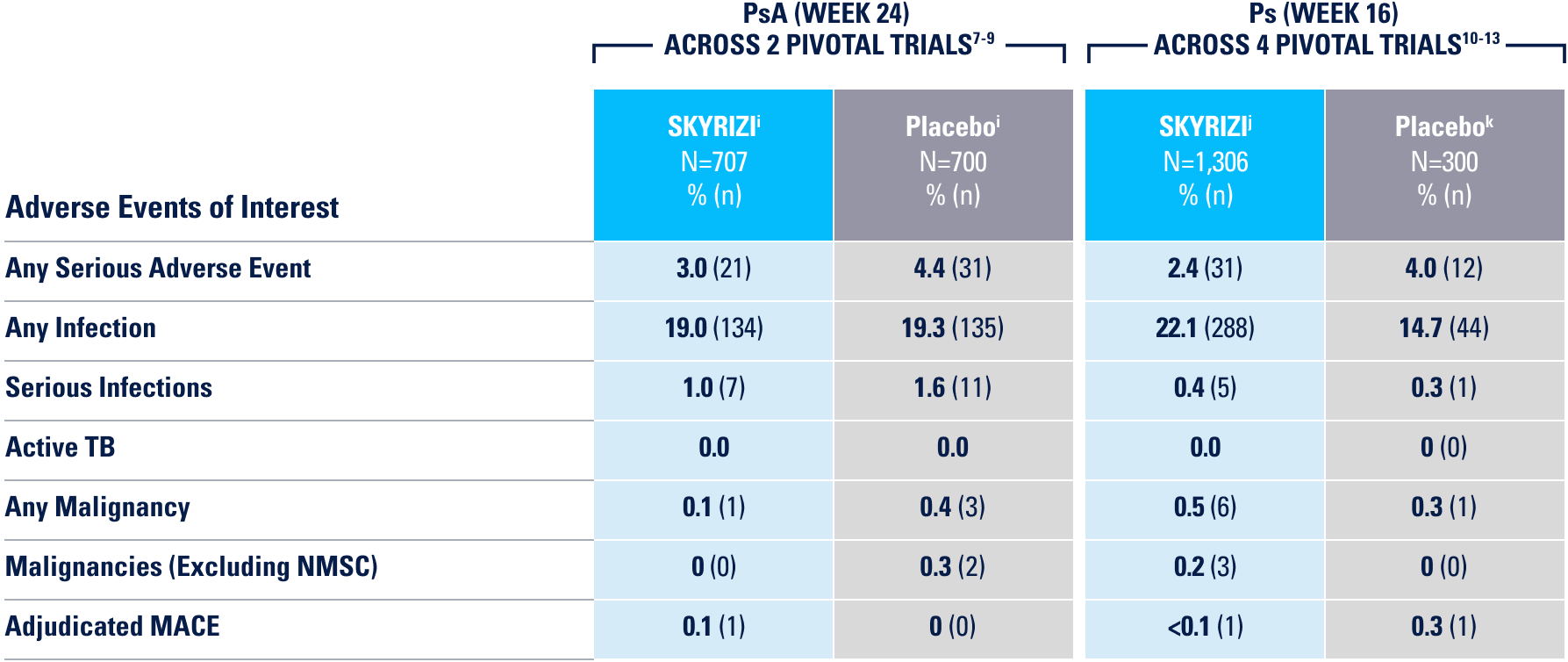
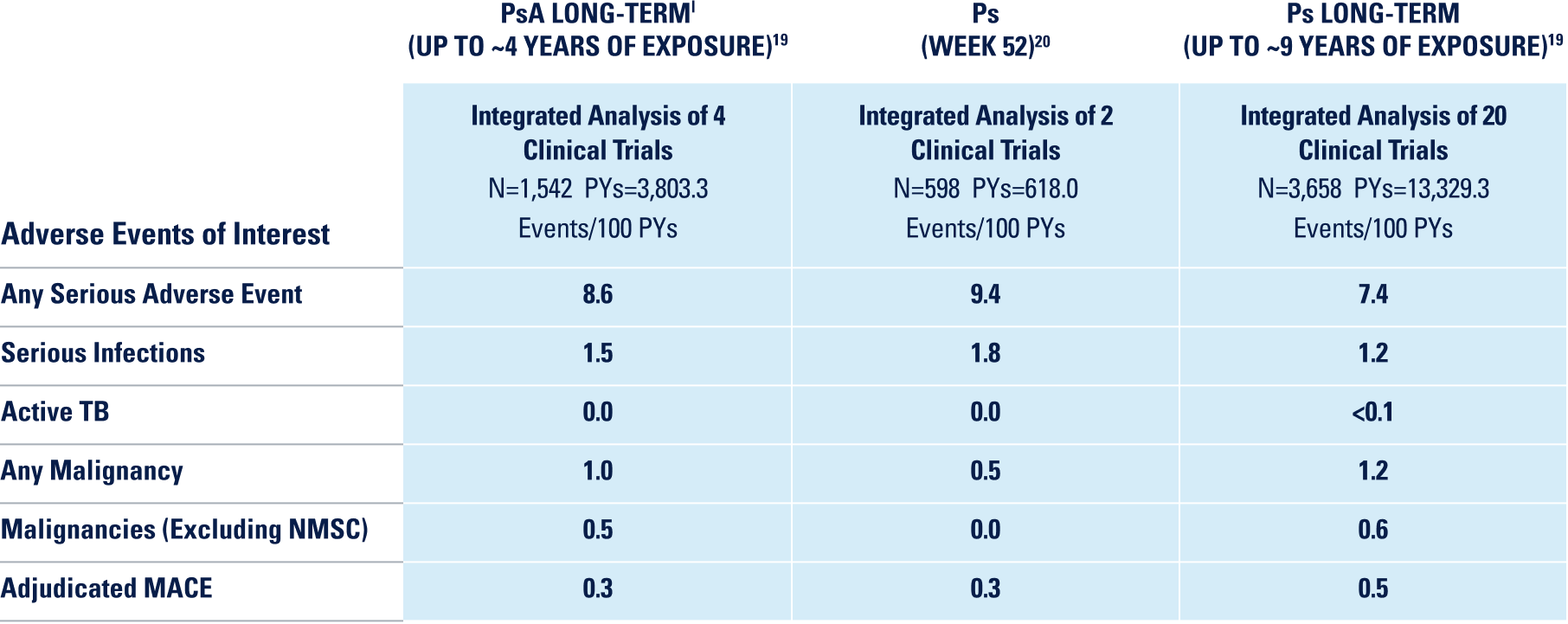
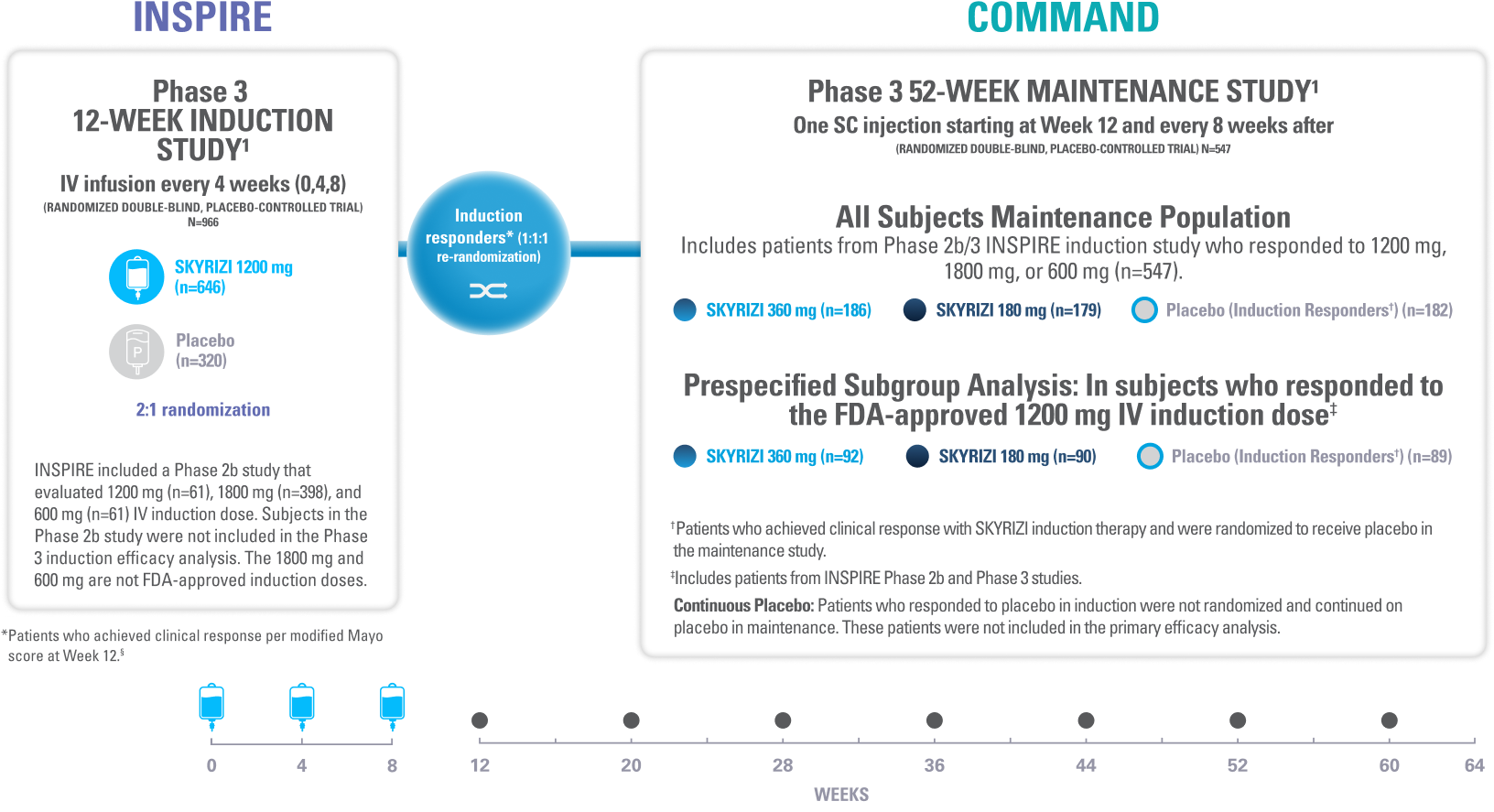
UP TO ~9 YEARS OF CLINICAL TRIAL EXPERIENCE ACROSS 4 INDICATIONS2
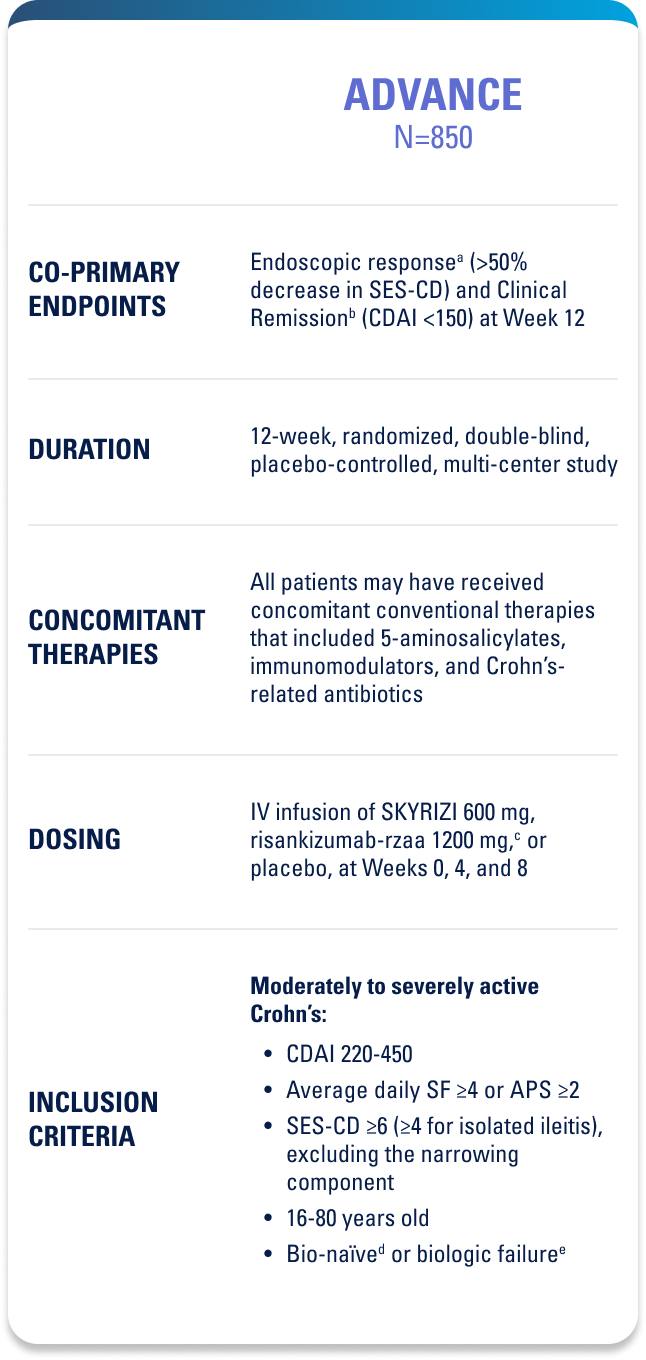
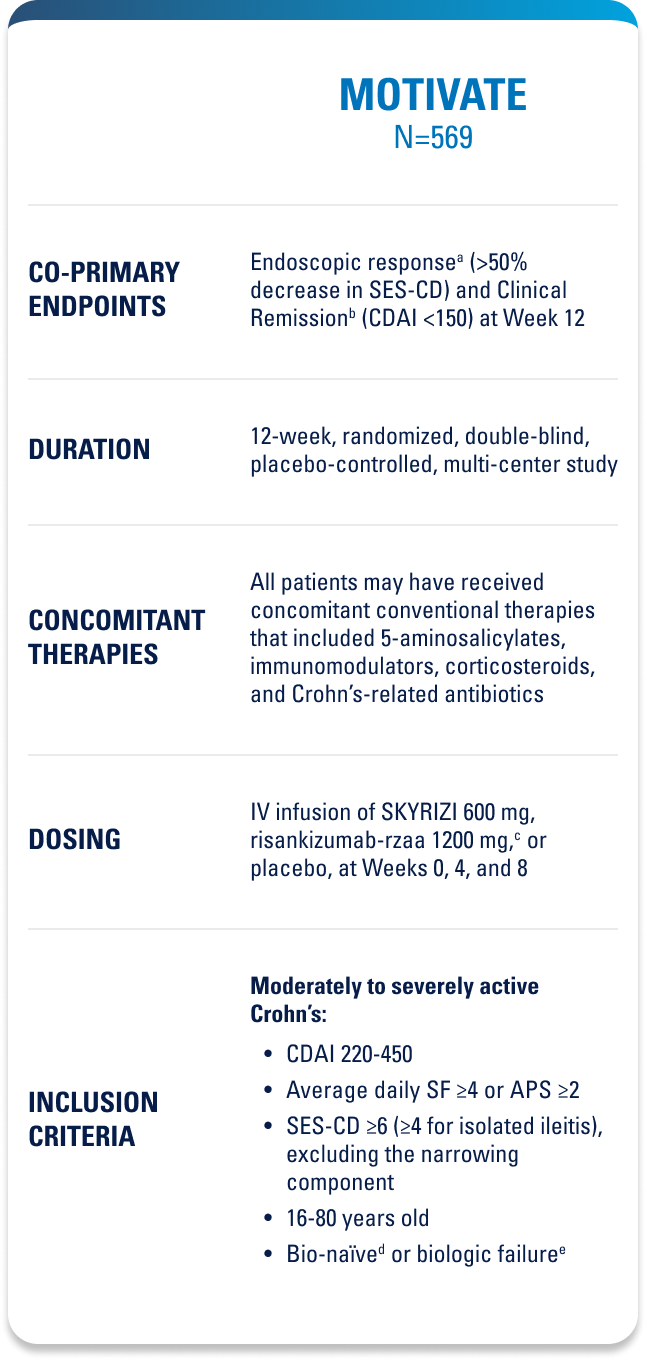
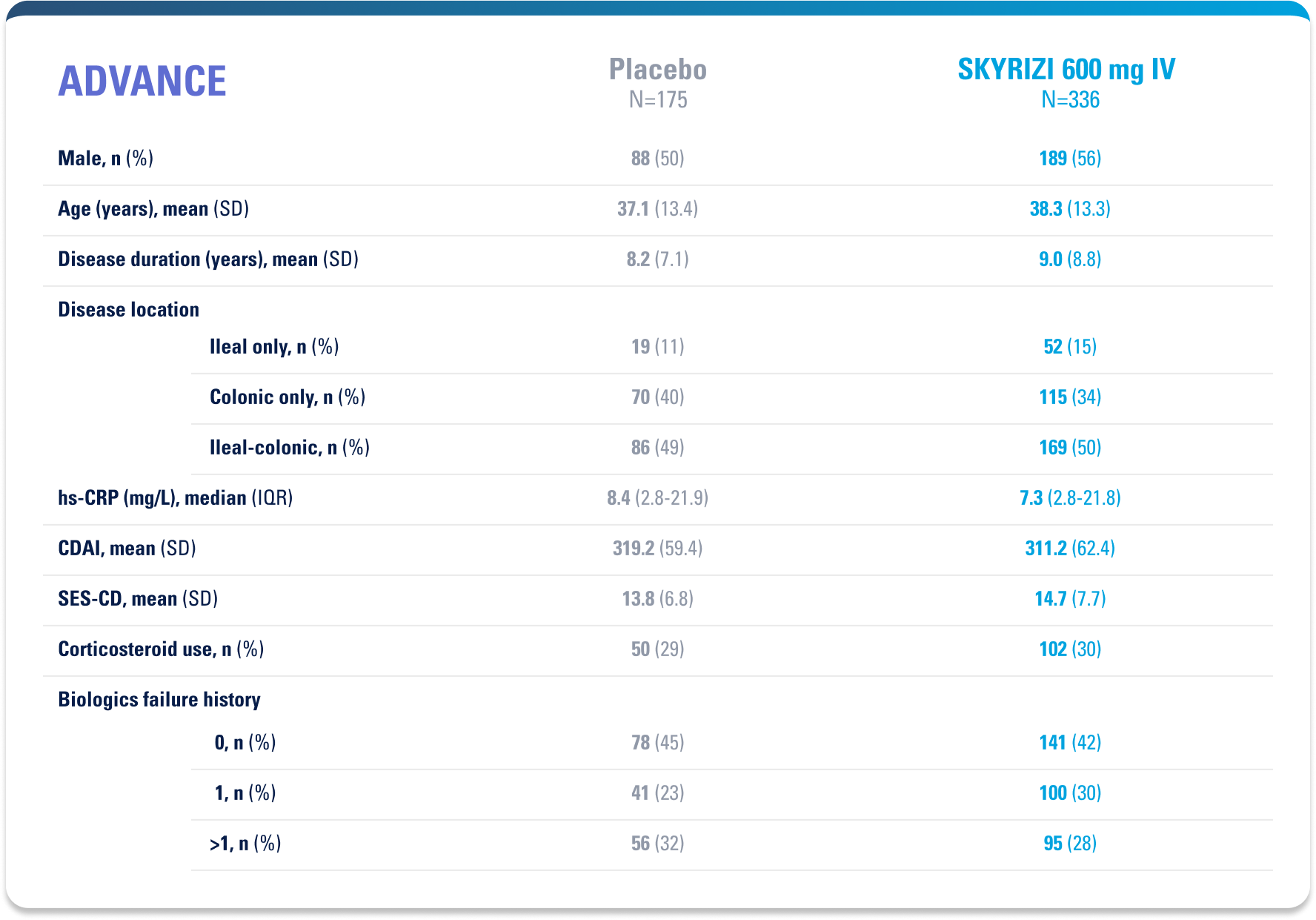
Approved for 4 Indications: Starting with plaque psoriasis (Ps), followed by psoriatic arthritis (PsA), Crohn’s disease, and ulcerative colitis (UC)1
*Safety data were evaluated for all patients receiving ≥1 dose of SKYRIZI from clinical trials, including open-label extension and dose-ranging studies.2
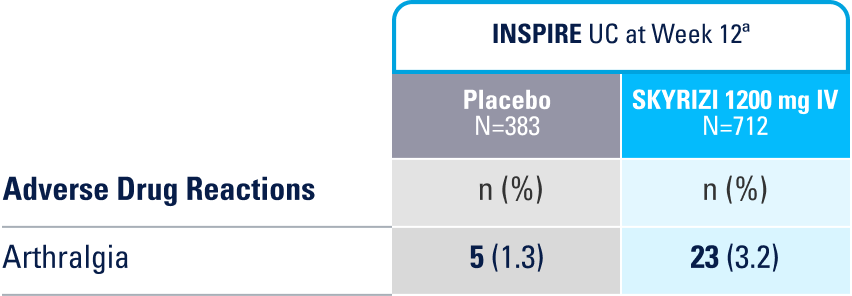
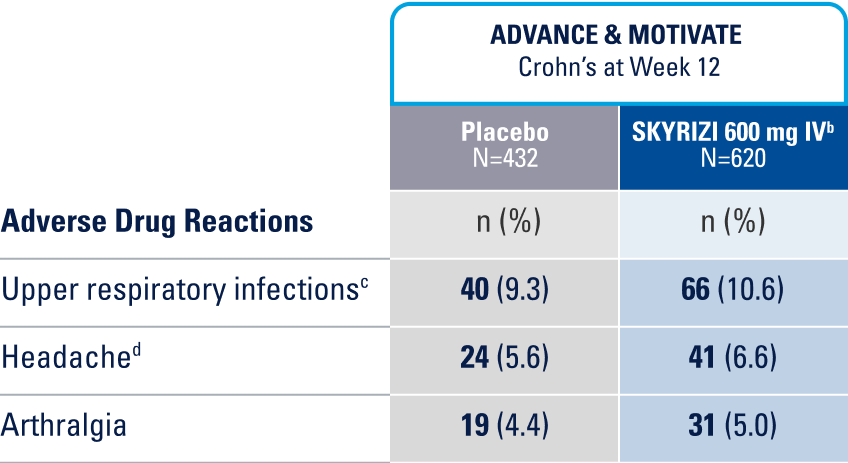
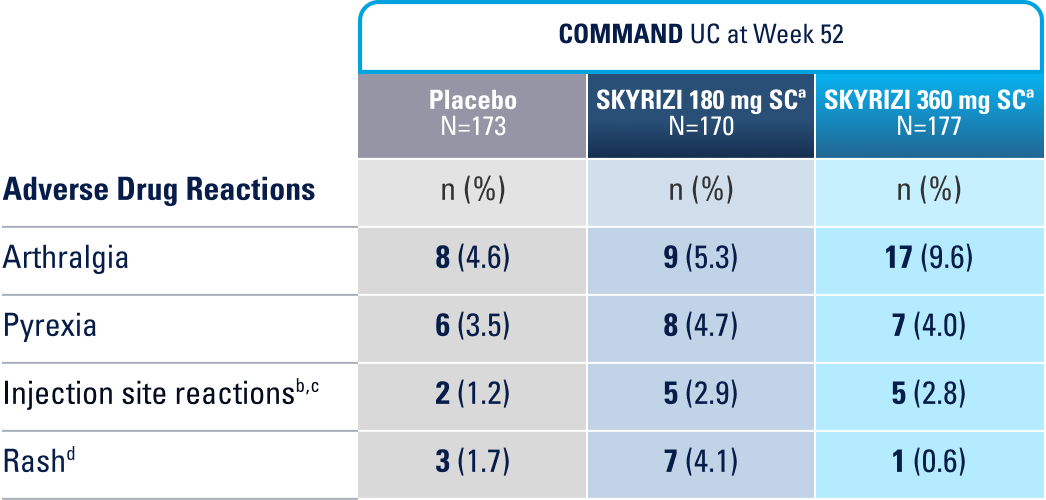
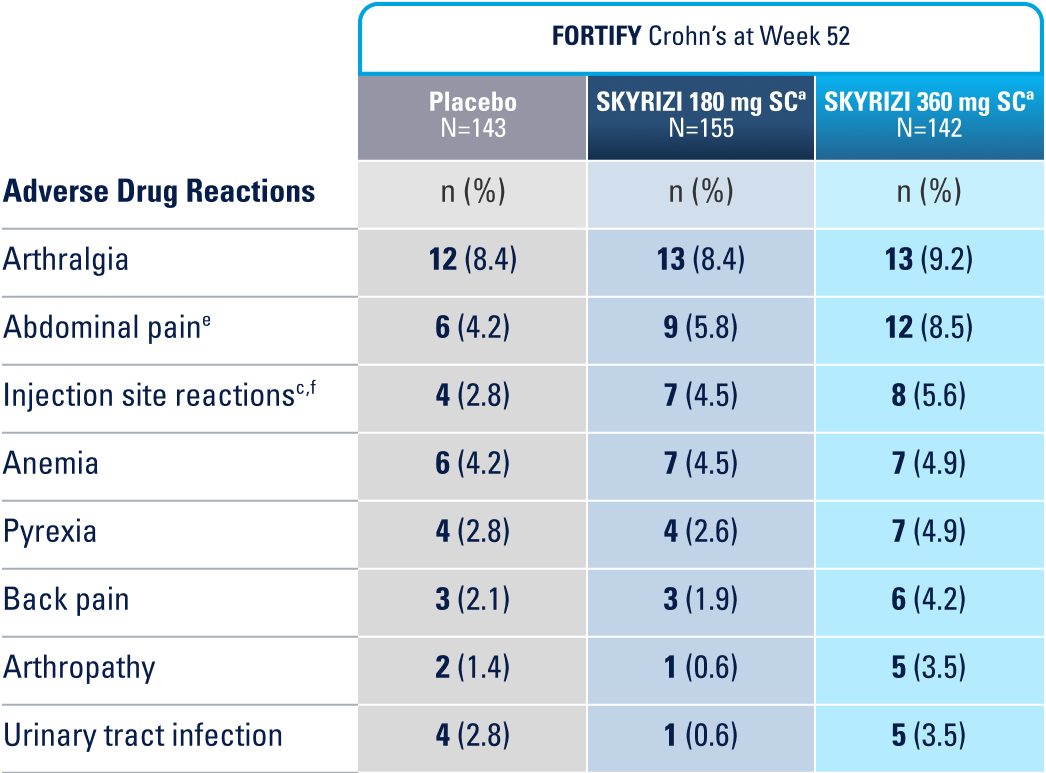
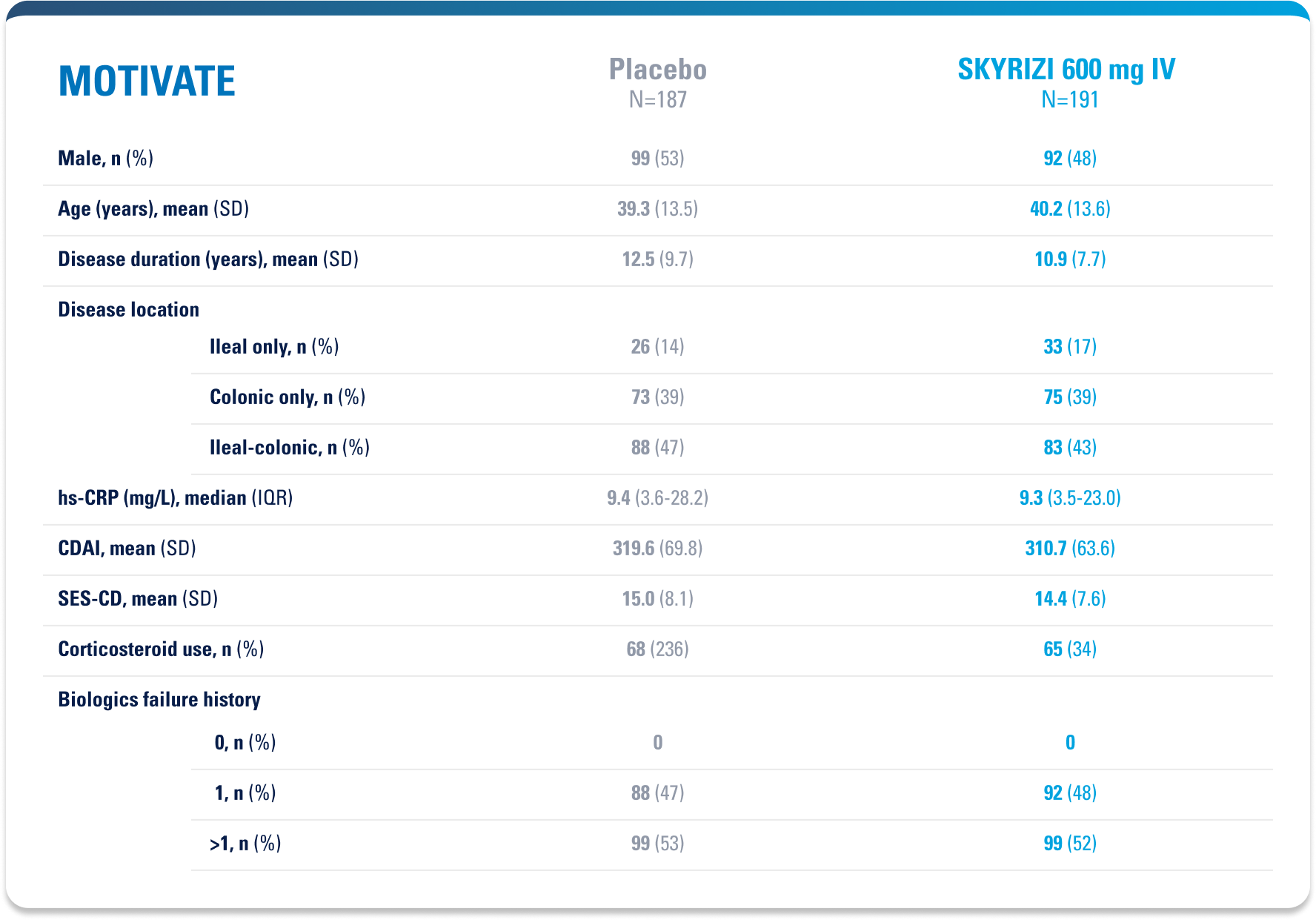
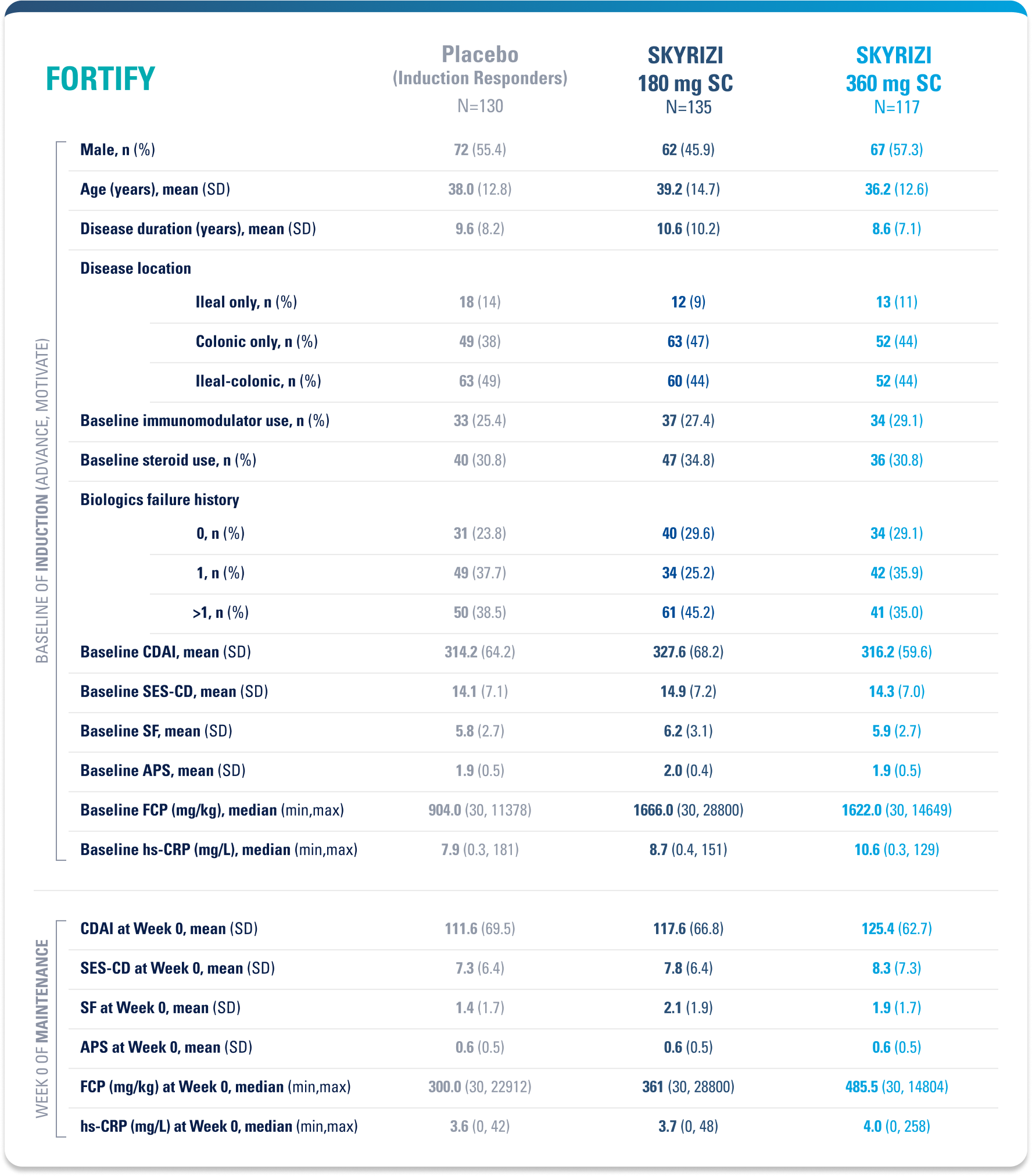
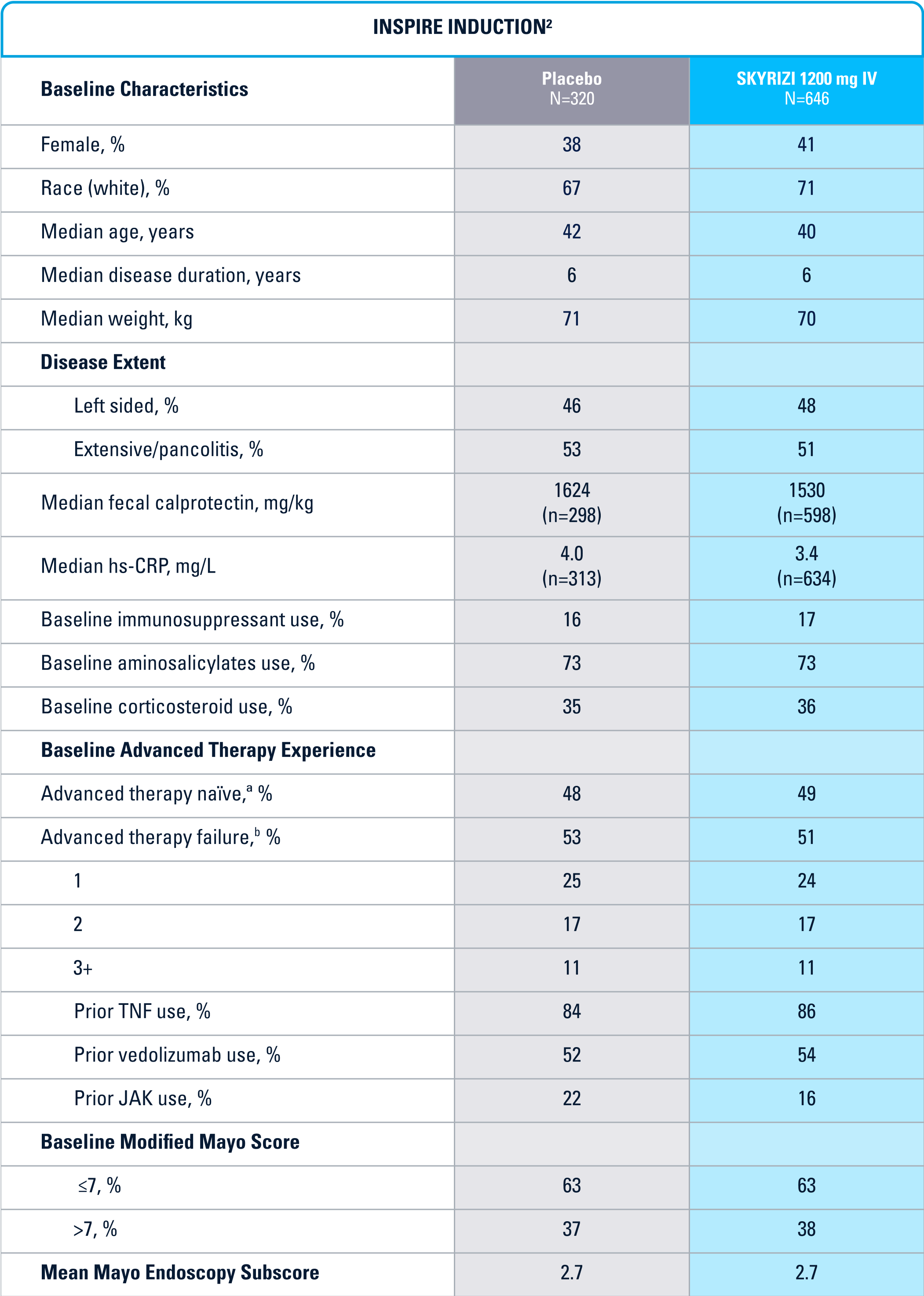
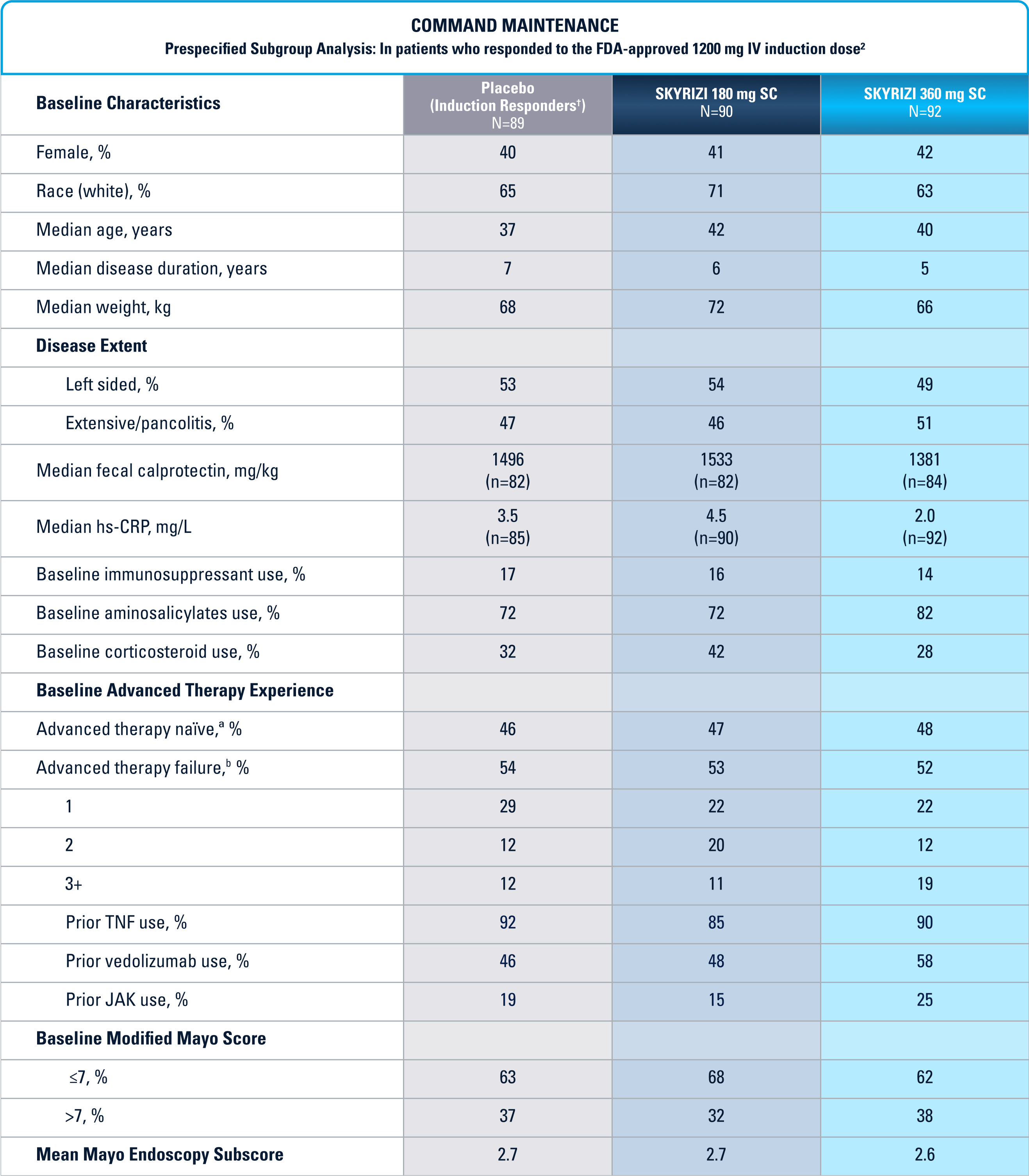
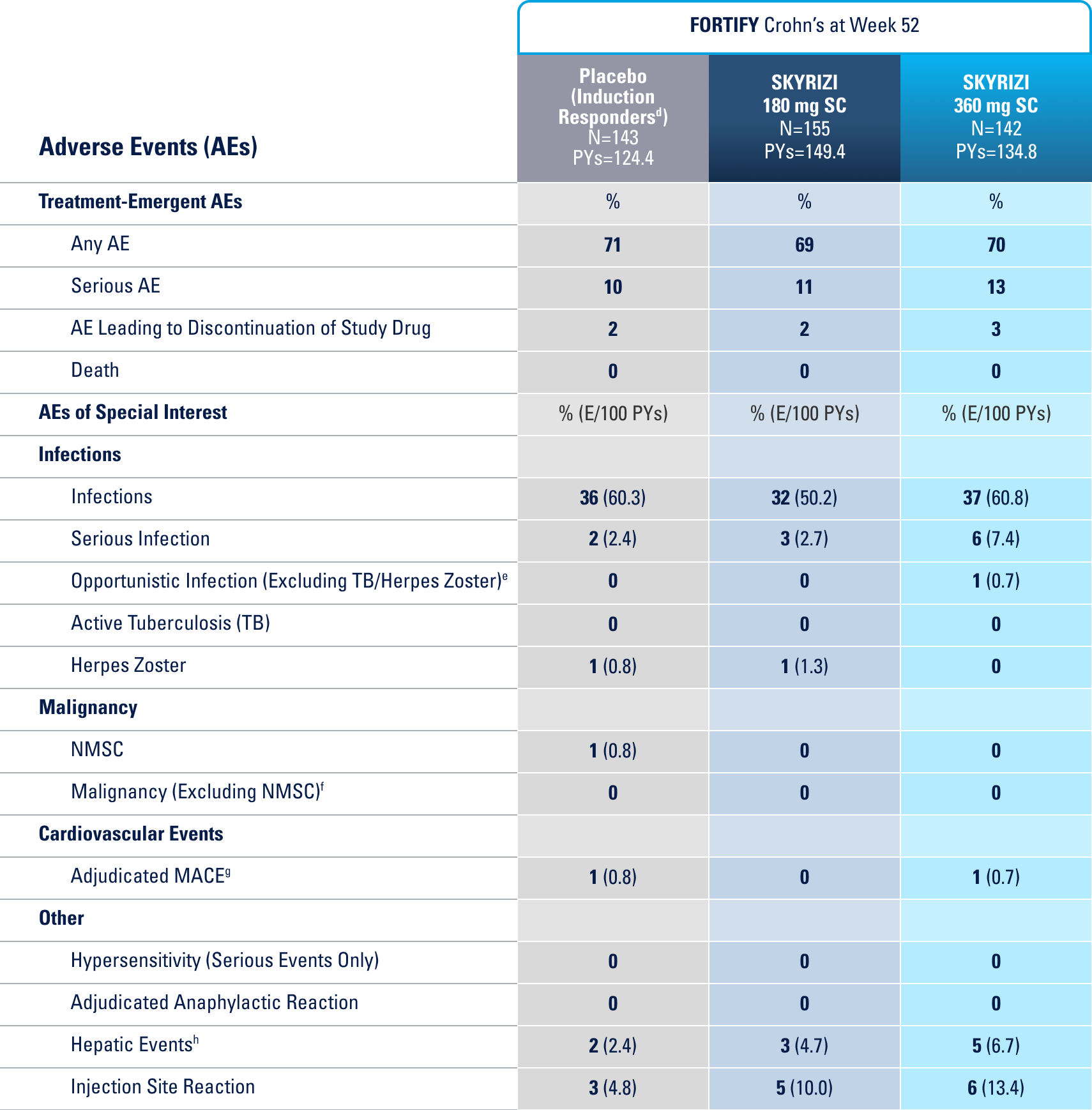
WELL-STUDIED SAFETY PROFILE IN UC & CROHN’S1,4-6
SWIPE TABLE TO VIEW MORE
SWIPE TABLE TO VIEW MORE
SWIPE TABLE TO VIEW MORE
SKYRIZI IS CONTRAINDICATED IN PATIENTS WITH A HISTORY OF SERIOUS HYPERSENSITIVITY REACTION TO RISANKIZUMAB-RZAA OR ANY OF THE EXCIPIENTS.1
IN THE TREATMENT OF CROHN’S, DRUG-INDUCED LIVER INJURY DURING INDUCTION HAS BEEN REPORTED. FOR THE TREATMENT OF CROHN’S DISEASE AND ULCERATIVE COLITIS, EVALUATE LIVER ENZYMES AND BILIRUBIN LEVELS AT BASELINE AND DURING INDUCTION, (12 WEEKS) OF TREATMENT. MONITOR THEREAFTER ACCORDING TO ROUTINE PATIENT MANAGEMENT.1
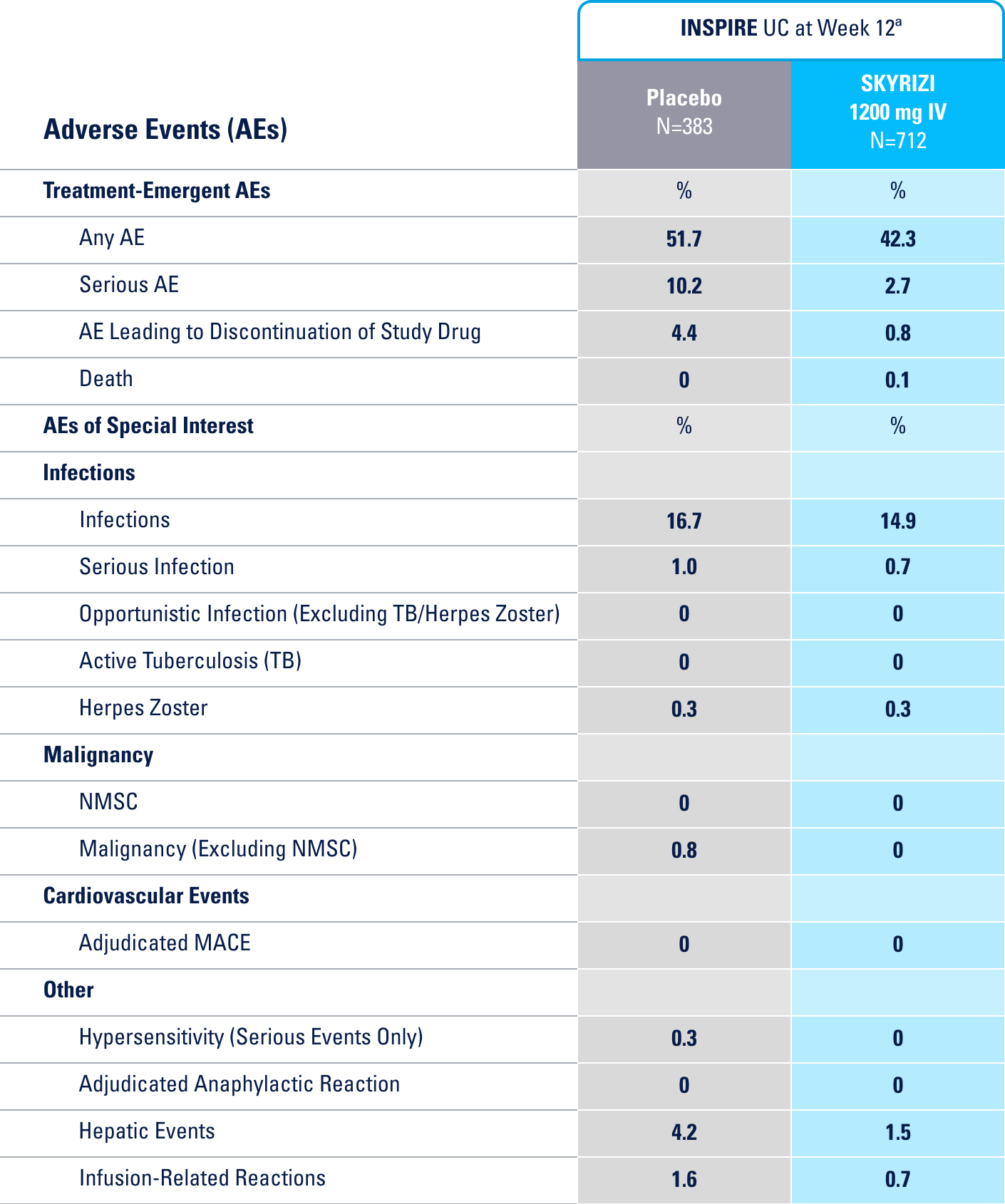
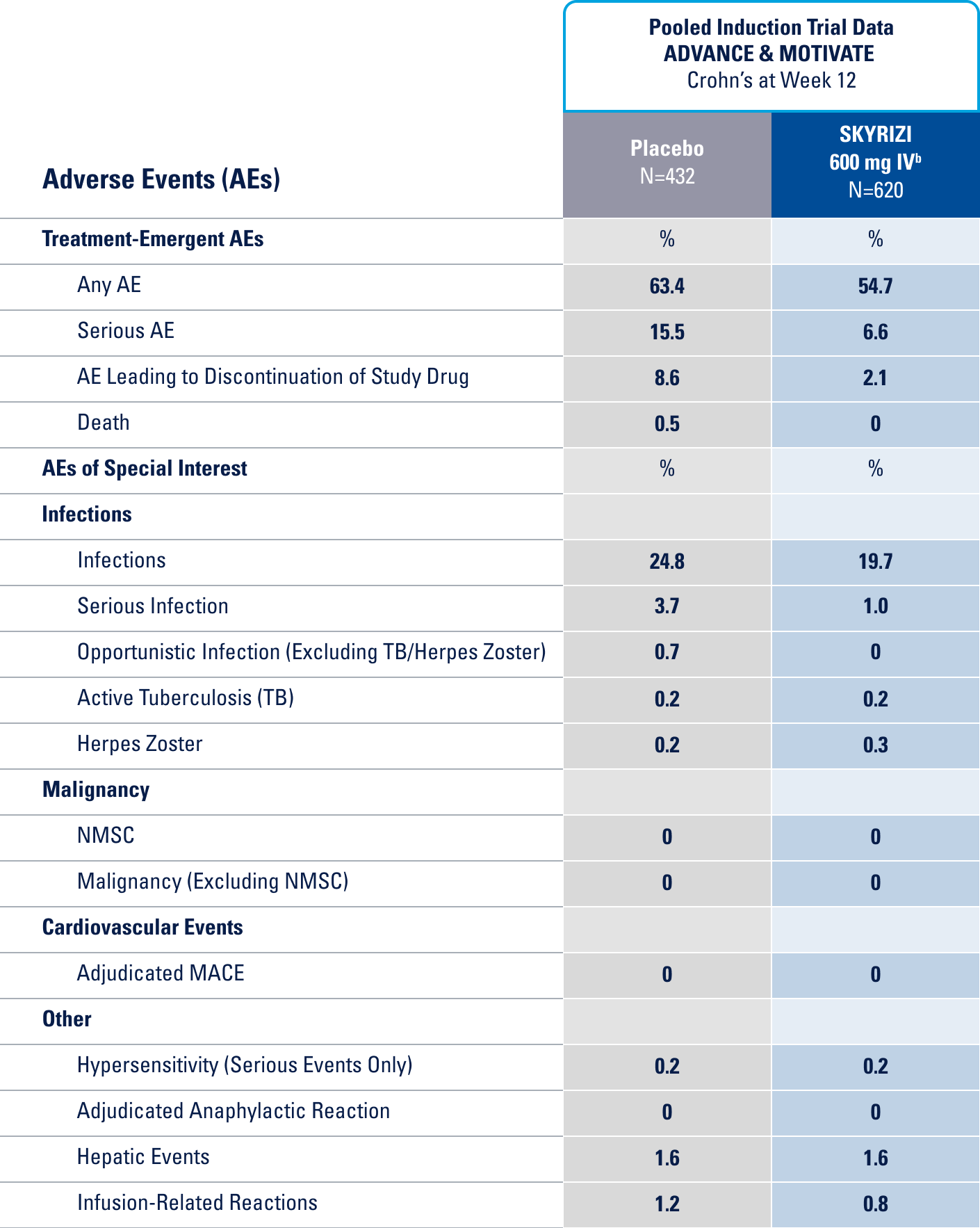
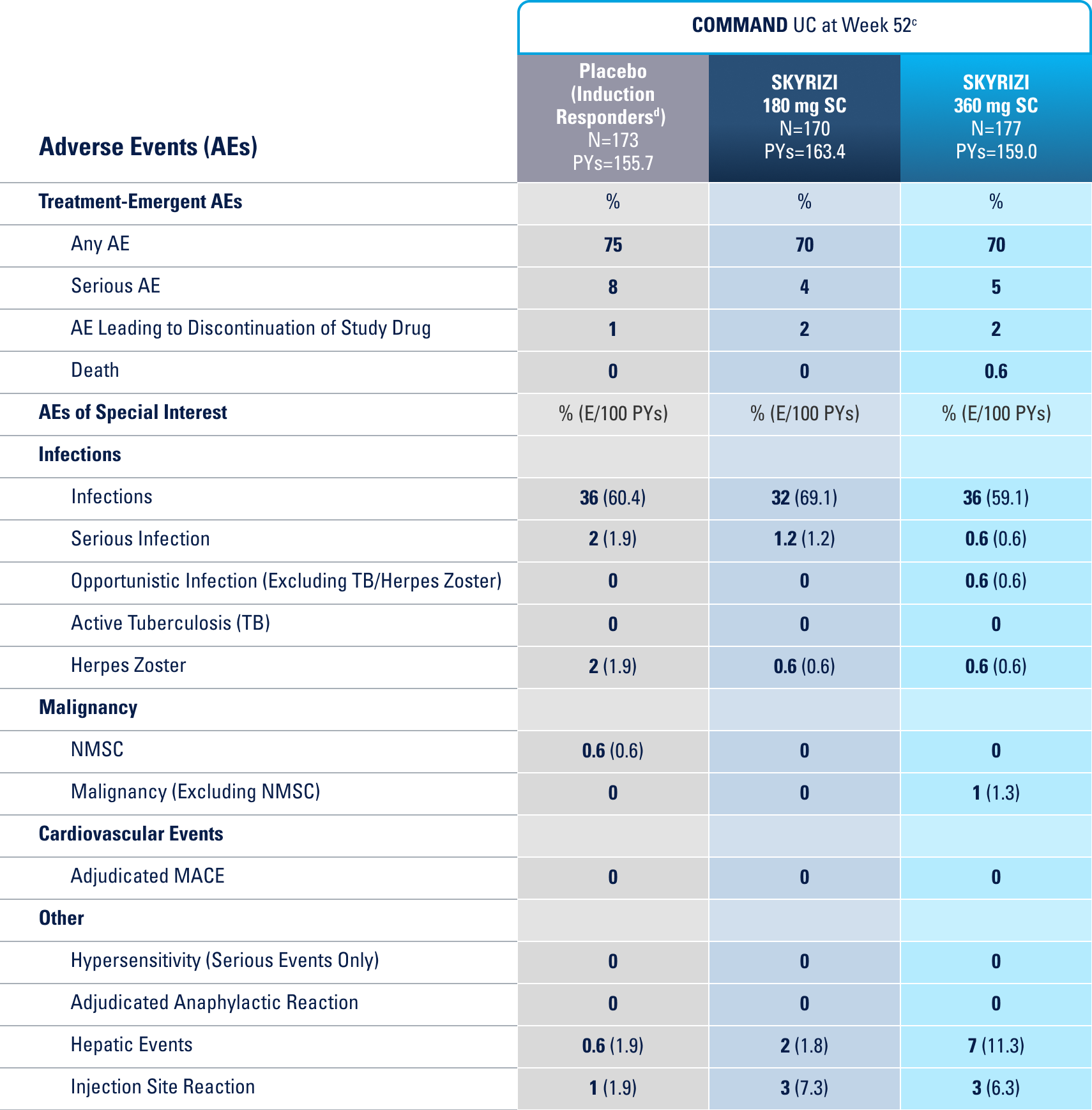
IN THE UC 12-WEEK INDUCTION STUDY, THE MOST COMMON ADVERSE REACTIONS (≥3% OF PATIENTS AND AT A HIGHER RATE THAN PLACEBO) INCLUDE ARTHRALGIA.1
IN THE CROHN'S 12-WEEK INDUCTION STUDY, THE MOST COMMON ADVERSE REACTIONS (>3% OF PATIENTS AND AT A HIGHER RATE THAN PLACEBO) INCLUDE UPPER RESPIRATORY INFECTIONS, HEADACHE, AND ARTHRALGIA.1
IN THE UC 52-WEEK MAINTENANCE STUDY, THE MOST COMMON ADVERSE REACTIONS (≥3% OF PATIENTS AND AT A HIGHER RATE THAN PLACEBO) INCLUDE ARTHRALGIA, PYREXIA, INJECTION SITE REACTIONS, AND RASH.1
IN THE CROHN'S 52-WEEK MAINTENANCE STUDY, THE MOST COMMON ADVERSE REACTIONS (>3% OF PATIENTS AND AT A HIGHER RATE THAN PLACEBO) INCLUDE ARTHRALGIA, ABDOMINAL PAIN, INJECTION SITE REACTIONS, ANEMIA, PYREXIA, BACK PAIN, ARTHROPATHY, AND URINARY TRACT INFECTION.1
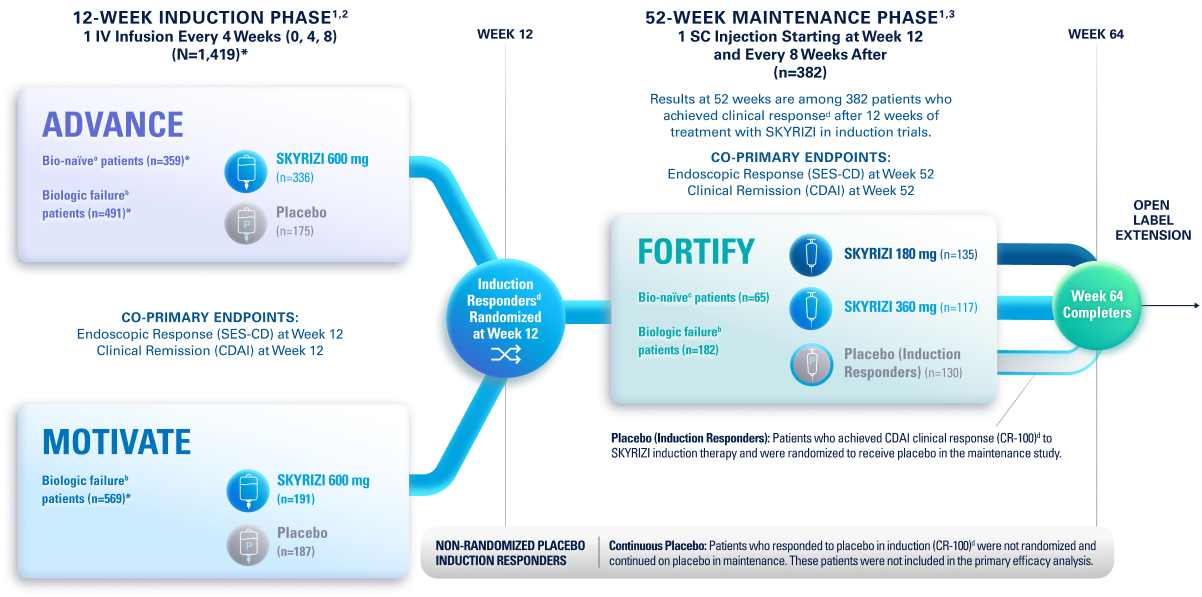
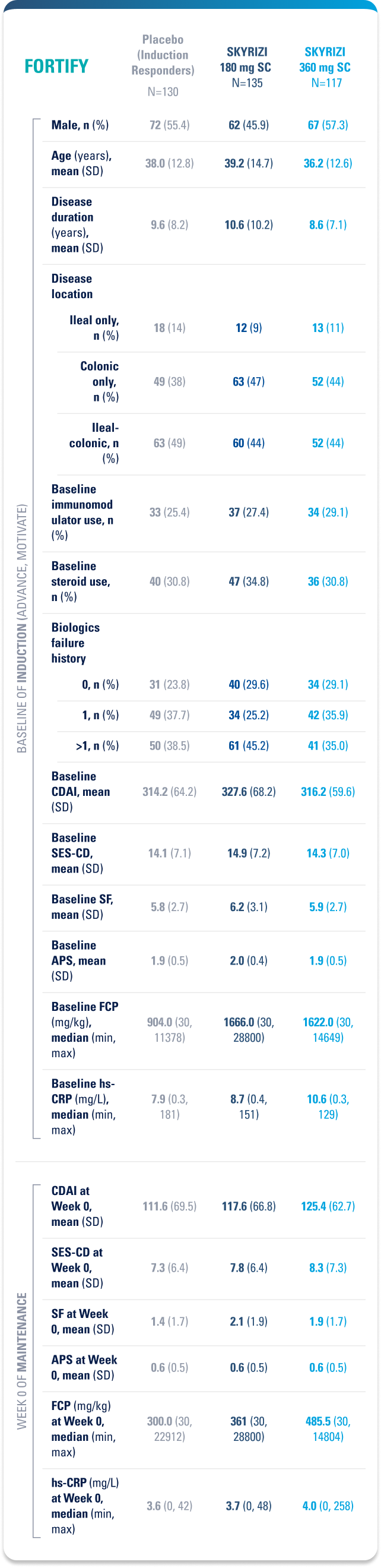
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR-100)¶ to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.
RECOMMENDED FOR YOU
LAB MONITORING FOR SKYRIZI
SKYRIZI treatment considerations in UC.
SKYRIZI ACCESS & REIMBURSEMENT
AbbVie can help your patients access SKYRIZI.
ENDOSCOPIC OUTCOMES WITH SKYRIZI1
An opportunity for endoscopic control.1