COMPACT SIZE
Small enough to fit in the palm of the hand
Click here for access and reimbursement resources for SKYRIZI.
The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active ulcerative colitis (UC) in adults.1
US-MULT-240253
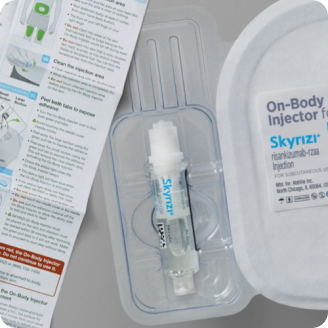
Single-dose SC injector with SKYRIZI prefilled cartridge1
SC=subcutaneous.
COMPACT SIZE
Small enough to fit in the palm of the hand
HANDS-FREE
While adhered to the abdomen or thigh and after activation, patients can do moderate physical activities such as walking, reaching, and bending
HIDDEN NEEDLE
Discreet injection so patients don’t see the needle
DOSE DELIVERED IN 5 MINUTES
Following preparation steps
AUDIO & VISUAL CUES
Notifies when full dose has been delivered
The SKYRIZI “Instructions for Use” includes the full set of detailed instructions on the preparation and administration of SKYRIZI. Instruct the patient to read before administration.1
PREPARE
Gather alcohol wipes, gauze pads or cotton balls, and sharps disposal container. Start the injection within 5 minutes after inserting the prefilled cartridge into the OBI.
PICK
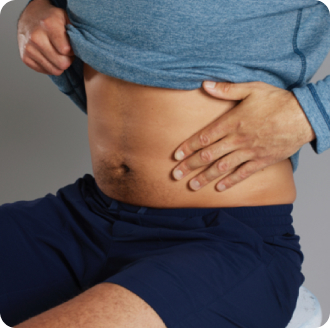
Pick a place on the thigh or abdomen to inject.

PEEL
Peel the 2 green pull tabs to reveal the adhesive without touching the needle.

PLACE
Place the OBI onto the skin. Ensure that the status light is visible.
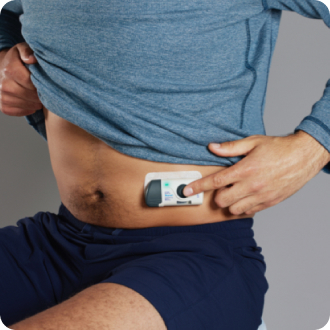
PRESS
Press and release the gray start button to begin. A flashing green light will appear. When the injection is complete, the green light goes solid.
ADMINISTRATION CONSIDERATIONS: SKYRIZI is intended for use under the guidance and supervision of a healthcare professional (HCP). Patients may self-inject SKYRIZI using the OBI with prefilled cartridge after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injetion technique of SKYRIZI according to the Instructions for Use.1
In a questionnaire, Crohn’s disease patients reported an experience with self-administration at home using the OBI after receiving training from their HCP2,3
97% (N=38/39) OF CROHN'S PATIENTS REPORTED

EASE OF USE
Was “Easy” or “Very Easy” to Use the OBI
SELF-INJECTION
Was “Very” or “Extremely Easy” to Self-Inject With the OBI
CONFIDENCE
Would be “Very” or “Extremely” Confident in Self-Injecting With the OBI at Home
DESCRIPTION: In an open-label, two-arm, multicenter study evaluating the SKRYIZI on-body injector (OBI) in adult patients with moderate-to-severe Crohn’s (N=46), analysis for patient experience was reported through questions within 6 domains of a validated Self-Injection Assessment Questionnaire (SIAQ). Data shown includes percent of patients that reported easy/very easy, very/extremely easy, or very/extremely confident for 3 questions within ease of use and satisfaction SIAQ domains at Week 8 (N=39). All data reported are observed. The SKYRIZI OBI was not evaluated in the primary analyses of the Phase 3 Clinical Trials in Crohn’s and UC.2,3
ADMINISTRATION CONSIDERATIONS: SKYRIZI is intended for use under the guidance and supervision of a healthcare professional (HCP). Patients may self-inject SKYRIZI using the OBI with prefilled cartridge after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of SKYRIZI according to the Instructions for Use.1
HCP=healthcare provider.
SKYRIZI COMPLETE SUPPORT PROGRAM
Providing support for your patients throughout the treatment journey.
SKYRIZI DOSING
Learn about SKYRIZI dosing and download a helpful guide.
SKYRIZI BILLING & CODING
Get information about billing and coding and download a helpful guide.
INDICATIONS AND IMPORTANT SAFETY INFORMATION FOR SKYRIZI® (risankizumab-rzaa)1 Indications Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults. Crohn's Disease: SKYRIZI is indicated for the treatment of moderately to severely active Crohn's disease in adults. Ulcerative Colitis: SKYRIZI is indicated for the treatment of moderately to severely active ulcerative colitis in adults. |
Important Safety Information
Hypersensitivity Reactions
SKYRIZI® (risankizumab-rzaa) is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab-rzaa or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of SKYRIZI. If a serious hypersensitivity reaction occurs, discontinue SKYRIZI and initiate appropriate therapy immediately.
Infection
SKYRIZI may increase the risk of infection. Do not initiate treatment with SKYRIZI in patients with a clinically important active infection until it resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing SKYRIZI. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, closely monitor and discontinue SKYRIZI until the infection resolves.
Tuberculosis (TB)
Prior to initiating treatment with SKYRIZI, evaluate for TB infection and consider treatment in patients with latent or active TB for whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SKYRIZI treatment. Do not administer SKYRIZI to patients with active TB.
Hepatotoxicity in Treatment of Inflammatory Bowel Disease
Drug-induced liver injury was reported in a patient with Crohn’s disease who was hospitalized for a rash during induction dosing of SKYRIZI. For the treatment of Crohn's disease and ulcerative colitis, evaluate liver enzymes and bilirubin at baseline and during induction (12 weeks); monitor thereafter according to routine patient management. Consider an alternate treatment for patients with evidence of liver cirrhosis. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Instruct your patient to seek immediate medical attention if they experience symptoms suggestive of hepatic dysfunction.
Administration of Vaccines
Avoid use of live vaccines in patients treated with SKYRIZI. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating SKYRIZI, complete all age-appropriate vaccinations according to current immunization guidelines.
Adverse Reactions
Most common (≥1%) adverse reactions associated with SKYRIZI in plaque psoriasis and psoriatic arthritis include upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
In psoriatic arthritis phase 3 trials, the incidence of hepatic events was higher with SKYRIZI compared to placebo.
Most common (>3%) adverse reactions associated with SKYRIZI in Crohn’s disease are upper respiratory infections, headache, and arthralgia in induction and arthralgia, abdominal pain, injection site reactions, anemia, pyrexia, back pain, arthropathy, and urinary tract infection in maintenance.
Most common (≥3%) adverse reactions associated with SKYRIZI in ulcerative colitis are arthralgia in induction, and arthralgia, pyrexia, injection site reactions, and rash in maintenance.
Lipid Elevations: Increases from baseline and increases relative to placebo were observed at Week 4 and remained stable to Week 12 in patients treated with SKYRIZI in Crohn’s disease. Lipid elevations observed in patients with ulcerative colitis were similar to those in Crohn's disease.
Dosage Forms and Strengths: SKYRIZI (risankizumab-rzaa) is available in a 150 mg/mL prefilled syringe and pen, a 600 mg/10 mL single-dose vial for intravenous infusion, and a 180 mg/1.2 mL or 360 mg/2.4 mL single-dose prefilled cartridge with on-body injector.
INDICATIONS
Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults.
Crohn's Disease: SKYRIZI is indicated for the treatment of moderately to severely active Crohn's disease in adults.
Ulcerative Colitis: SKYRIZI is indicated for the treatment of moderately to severely active ulcerative colitis in adults.
Please see Full Prescribing Information.
US-SKZG-240258
REFERENCES