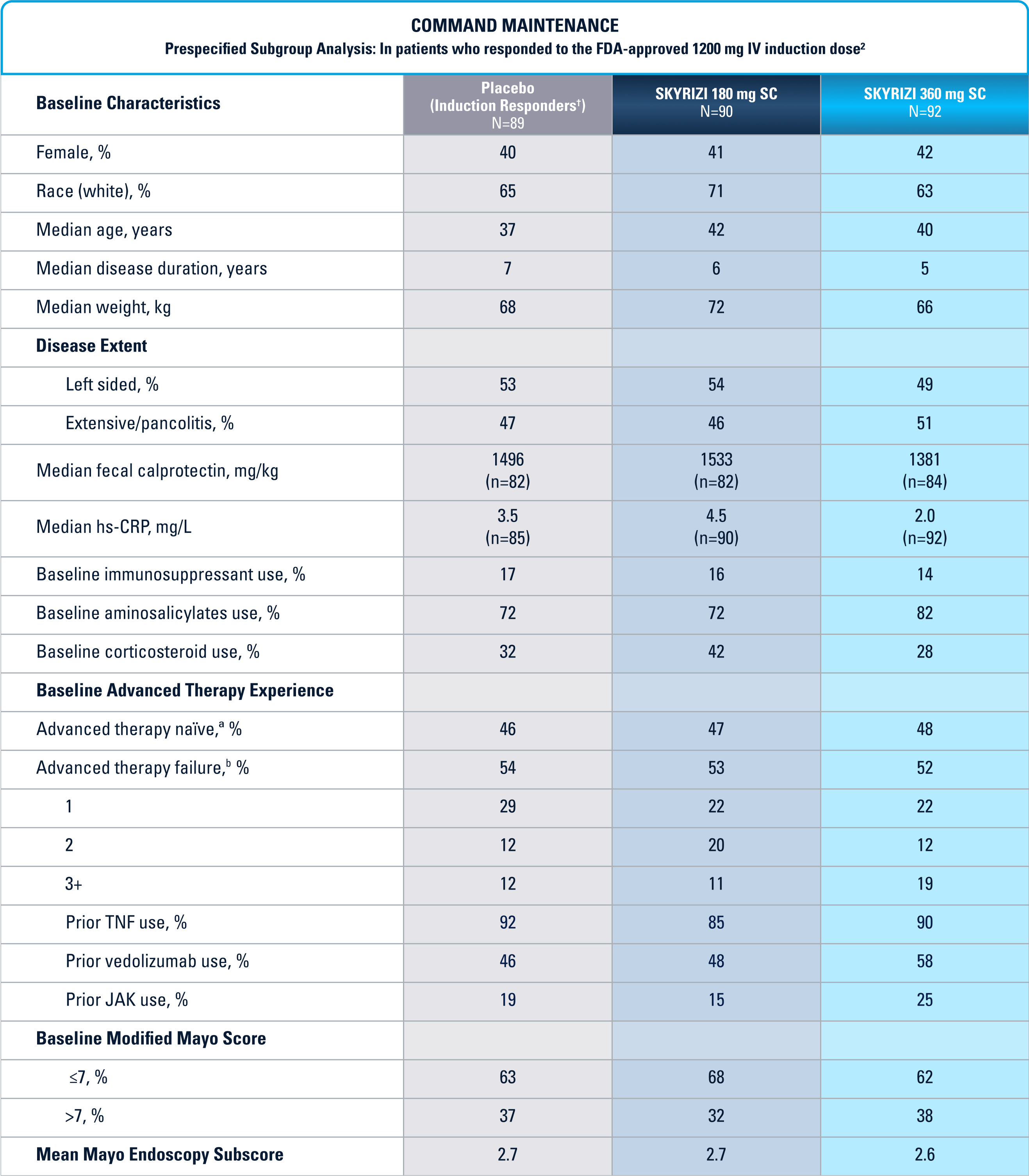
Prespecified subgroup analysis: In patients who responded to the FDA-approved 1200 mg IV induction dose2
EFFICACY
ON THIS PAGE:
SKYRIZI MET ITS PRIMARY ENDPOINTS1
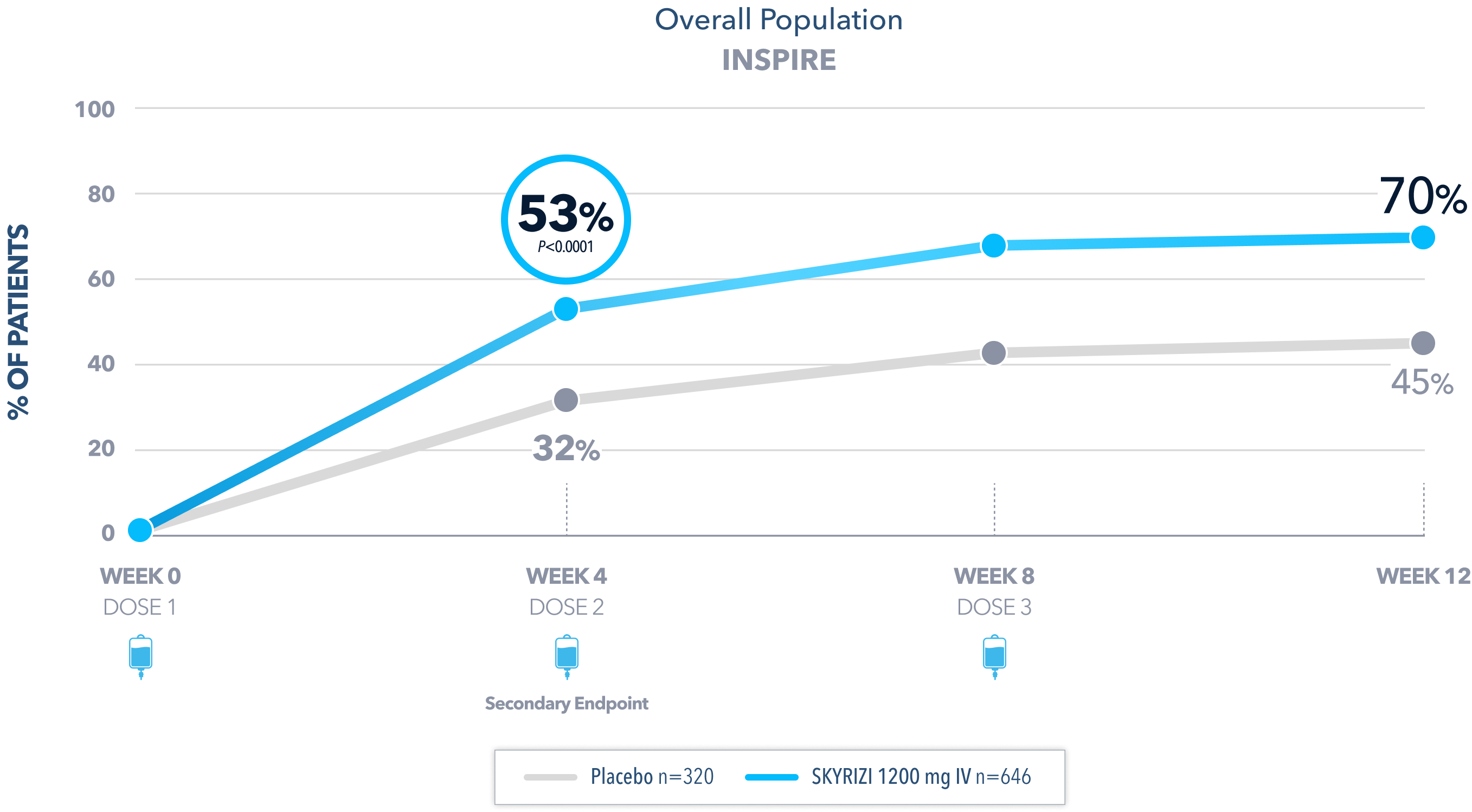
INSPIRE PHASE 3 INDUCTION
Clinical Remission* at Week 12:
8% Placebo (n=320)
COMMAND ALL SUBJECTS MAINTENANCE POPULATION†
Clinical Remission* at Week 52:
26% Placebo (Induction Responders‡) (n=182)
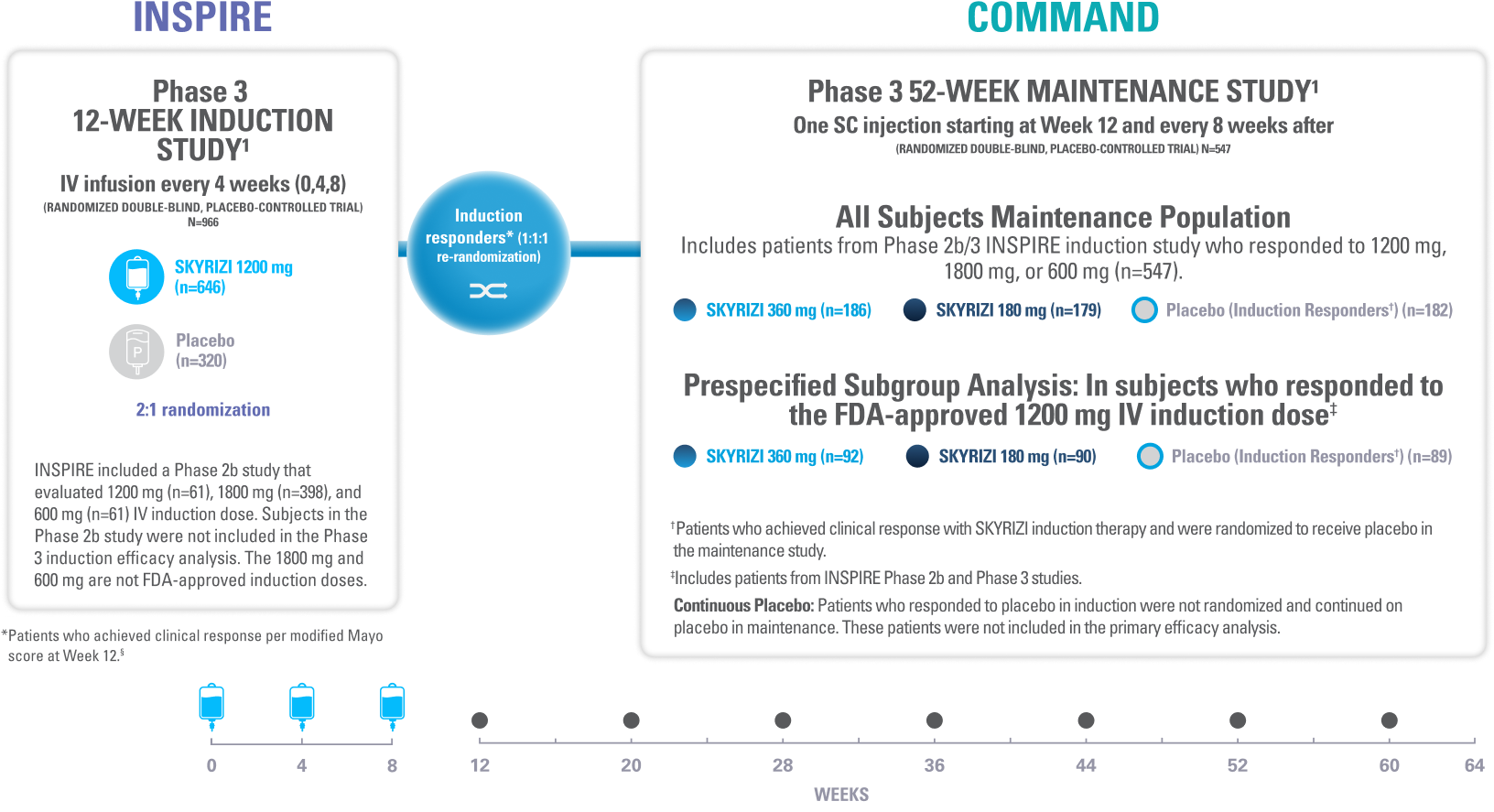
†Includes patients who responded to 1800 mg, 1200 mg, or 600 mg in the Phase 2b/3 induction study; 600 mg and 1800 mg are not FDA-approved induction doses.1
All P-values are SKYRIZI treatment arms vs placebo.
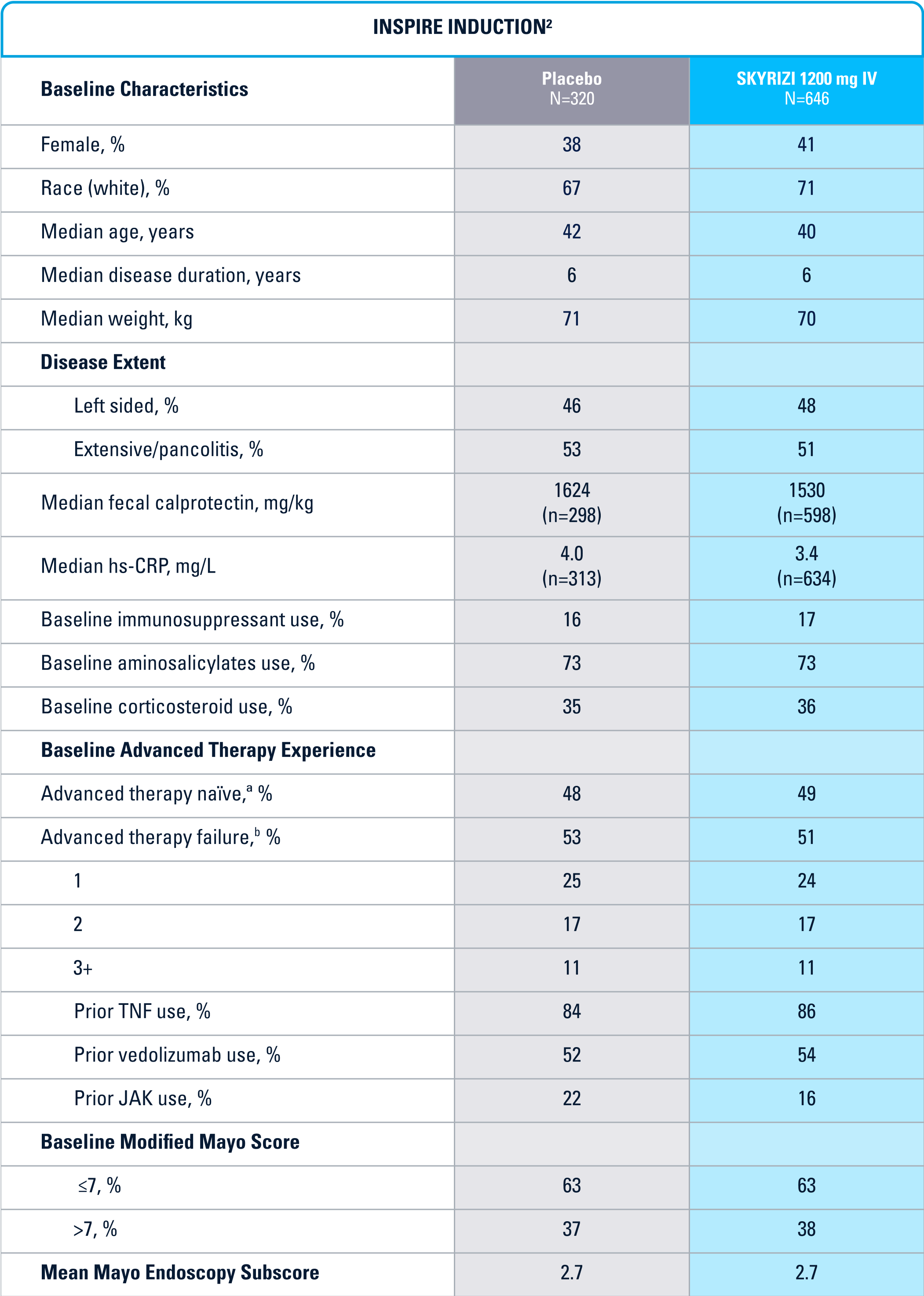
INSPIRE Induction (N=966) was a Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SKYRIZI 1200 mg IV, at Weeks 0, 4, and 8 vs placebo over 12 weeks in adult patients with moderate to severe UC. The primary endpoint was clinical remission* at Week 12.1
COMMAND Maintenance (N=547) was a Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SKYRIZI 180 mg or 360 mg SC vs placebo up to 52 weeks in adult patients who were clinical responders to one of three SKYRIZI induction regimens consisting of 600 mg, 1200 mg or 1800 mg IV (the 600 mg and 1800 mg IV are not FDA-approved induction regimens) in the INSPIRE Phase 2b or Phase 3 studies. The primary endpoint was clinical remission* at Week 52. COMMAND also included a prespecified subgroup analysis inclusive only of patients who responded to the FDA-approved induction dosing of SKYRIZI 1200 mg IV (N=271).1
*Clinical remission in UC is defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and endoscopic subscore ≤1 without friability.1
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
IV=intravenous; SC=subcutaneous.
ENDOSCOPIC DATA
Endoscopic Improvement§ at Week 522
(Endoscopy subscore of 0 or 1, without friability)
Prespecified subgroup analysis: In patients who responded to the FDA-approved 1200 mg IV induction dose2
Limitations of prespecified subgroup analysis: Results shown are only among patients who responded to the FDA-approved 1200 mg IV induction dose. Excluded are those who received unapproved induction doses (600 mg or 1800 mg). Data were not controlled for multiplicity. No conclusions or statistical inferences can be made.2
All Subjects¶ Maintenance Population1
At Week 52, proportion of patients who achieved endoscopic improvement§ (secondary endpoint):
51% SKYRIZI 180 mg SC (n=179; P<0.001)
48% SKYRIZI 360 mg SC (n=186; P<0.001)
31% Placebo (Induction Responders‡) (n=182)
All P-values are SKYRIZI treatment arms vs placebo (induction responders).
¶Patients who responded to 1800 mg, 1200 mg, or 600 mg in Phase 2b/3 induction study.1
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
§Endoscopic improvement in UC is defined as Mayo endoscopic subscore of 0 or 1 without friability.1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, at the discretion of the investigator.
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
EXAMPLE OF ENDOSCOPY SUBSCORES3
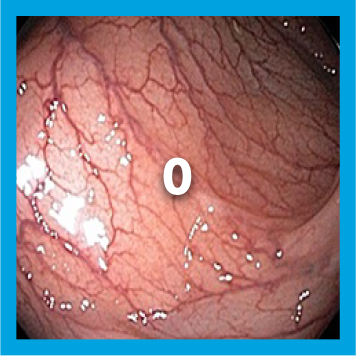
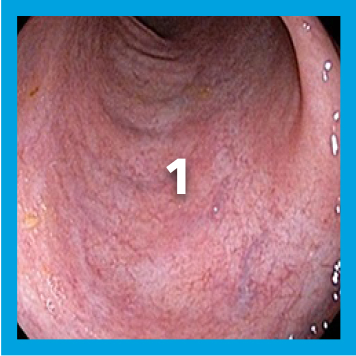
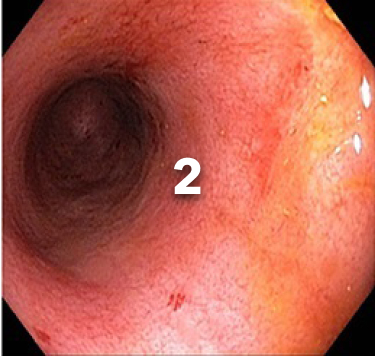
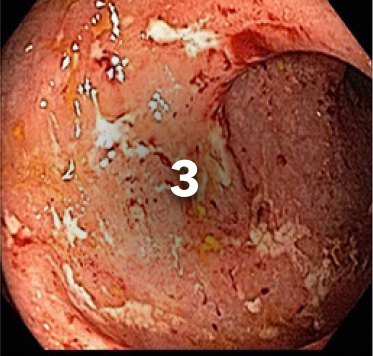
Patient baseline characteristics at induction include4:
~70%
ENDOSCOPIC SUBSCORE 3
~30%
ENDOSCOPIC SUBSCORE 2
IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate; SC=subcutaneous.
Endoscopic Remission# at Week 522
(Endoscopy subscore of 0)
Prespecified subgroup analysis: In patients who responded to the FDA-approved 1200 mg IV induction dose2
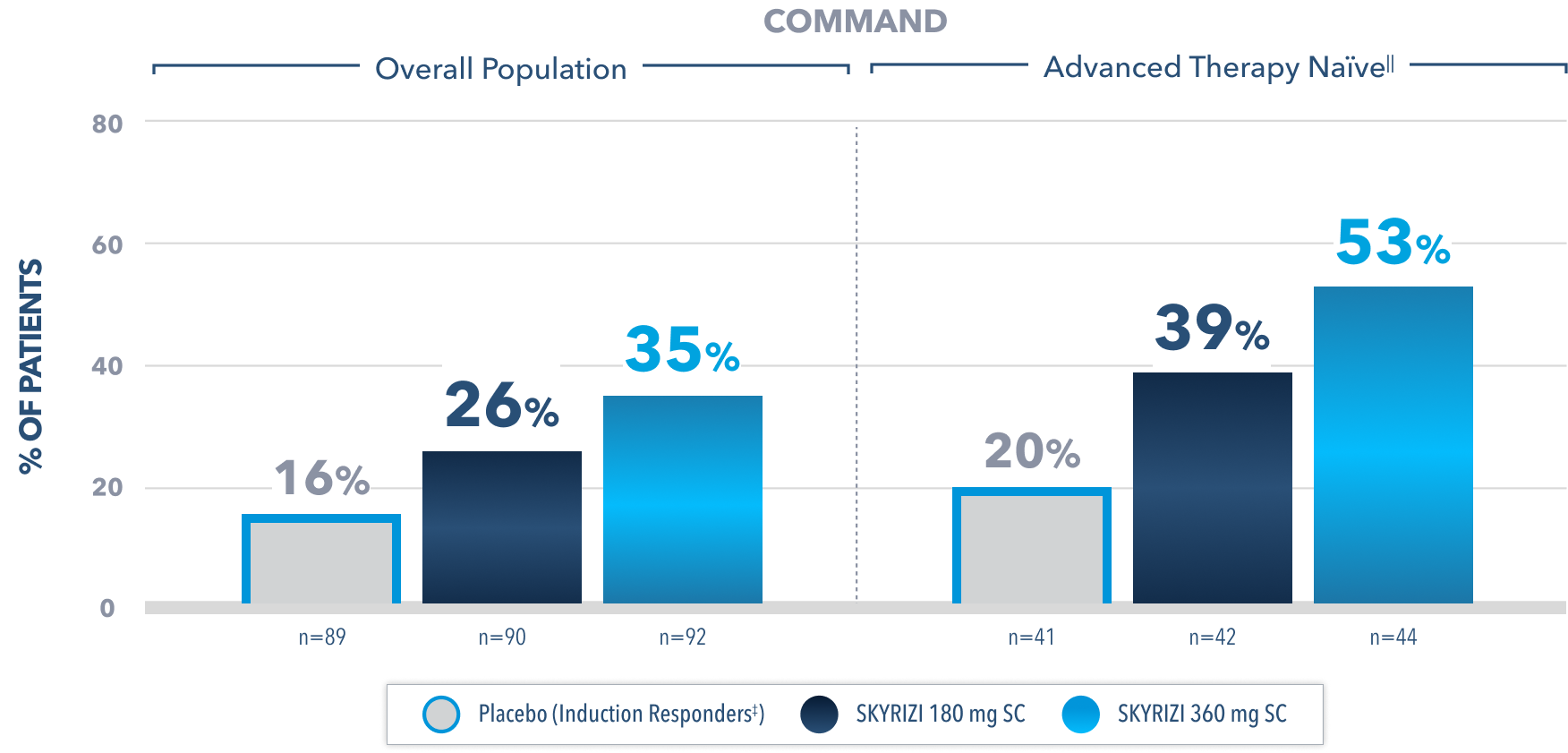
Limitations of prespecified subgroup analysis: Results shown are only among patients who responded to the FDA-approved 1200 mg IV induction dose. Excluded are those who received unapproved induction doses (600 mg or 1800 mg). Data were not controlled for multiplicity. No conclusions or statistical inferences can be made.2
All Subjects¶ Maintenance Population1,2
At Week 52, proportion of patients who achieved endoscopic remission# (secondary endpoint):
23% SKYRIZI 180 mg SC (n=179; P<0.05)
24% SKYRIZI 360 mg SC (n=186; P<0.05)
15% Placebo (Induction Responders‡) (n=182)
All P-values are SKYRIZI treatment arms vs placebo (induction responders).
¶Patients who responded to 1800 mg, 1200 mg, or 600 mg in Phase 2b/3 induction study.1
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
#Endoscopic remission in UC is defined as Mayo endoscopic subscore of 0.1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, at the discretion of the investigator.
EXAMPLE OF ENDOSCOPY SUBSCORE3

ES=0 is endoscopic remission
Normalization of endoscopic appearance of the mucosa
Patient baseline characteristics at induction include4:
~70%
ENDOSCOPIC SUBSCORE 3
~30%
ENDOSCOPIC SUBSCORE 2
ES=endoscopy subscore; IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate; SC=subcutaneous.
Histo-Endoscopic Mucosal Improvement** at Week 522
(Endoscopy subscore of 0 or 1 without friability and histologic improvement with Geboes score ≤3.1)
Prespecified subgroup analysis: In patients who responded to the FDA-approved 1200 mg IV induction dose2
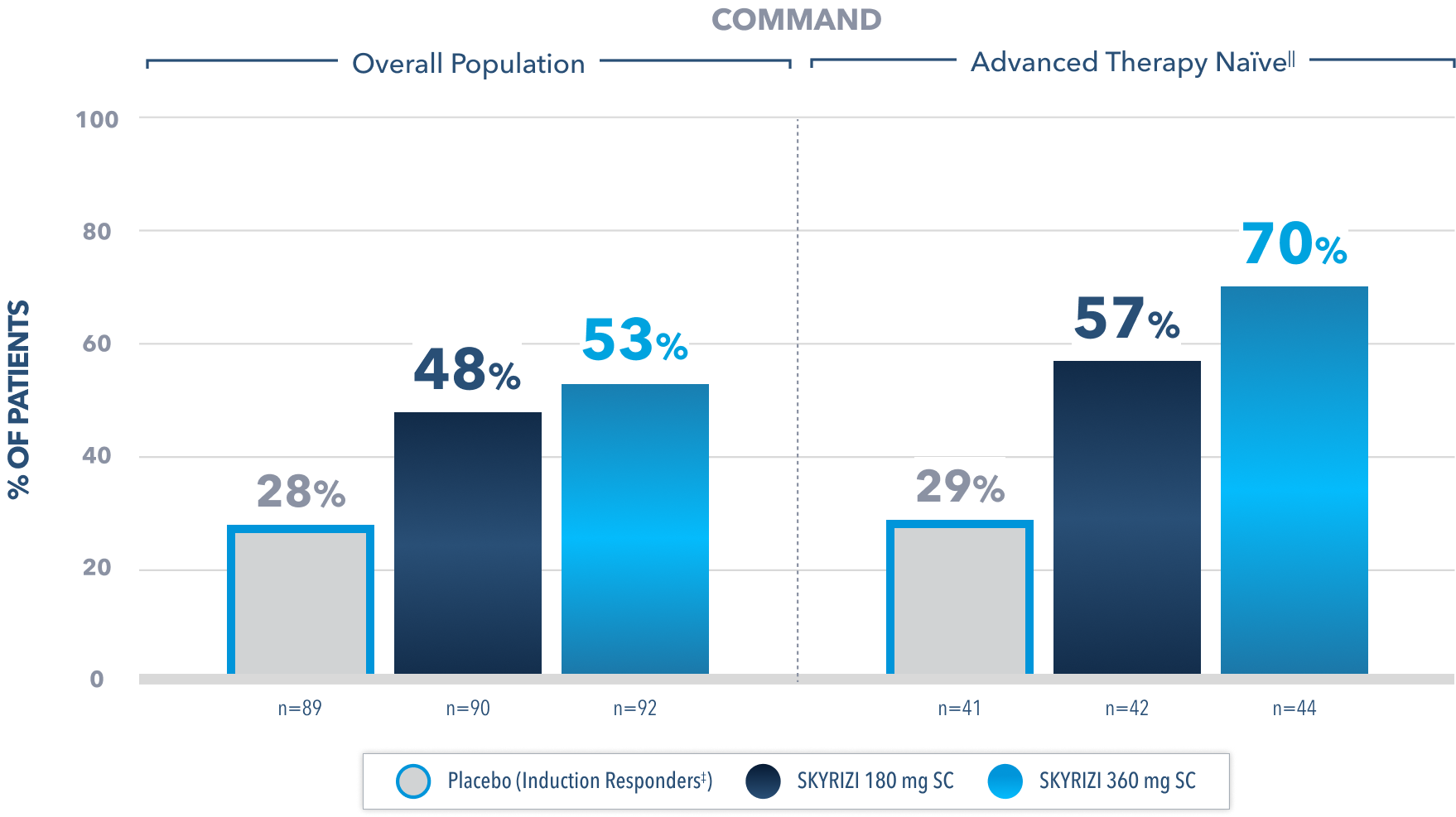
Limitations of prespecified subgroup analysis: Results shown are only among patients who responded to the FDA-approved 1200 mg IV induction dose. Excluded are those who received unapproved induction doses (600 mg or 1800 mg). Data were not controlled for multiplicity. No conclusions or statistical inferences can be made.2
All Subjects¶ Maintenance Population1
At Week 52, proportion of patients who achieved histo-endoscopic musocal improvement** (secondary endpoint):
43% SKYRIZI 180 mg SC (n=179; P<0.001)
42% SKYRIZI 360 mg SC (n=186; P<0.001)
24% Placebo (Induction Responders‡) (n=182)
All P-values are SKYRIZI treatment arms vs placebo (induction responders).
¶Patients who responded to 1800 mg, 1200 mg, or 600 mg in Phase 2b/3 induction study.1
The relationship between this endpoint to disease progression and long-term outcomes was not evaluated.
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
**Histo-endoscopic mucosal improvement in UC is defined as Mayo endoscopy subscore of 0 or 1 without friability and Geboes score ≤3.1 (neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue).1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, depending on the extent of disease at study entry, and histology results are based on a set of 2 biopsies.
IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate; SC=subcutaneous.
REMISSION DATA
Clinical Remission* at Week 522
(Composite of rectal bleeding, stool frequency, and endoscopy subscores)
Prespecified subgroup analysis: In patients who responded to the FDA-approved 1200 mg IV induction dose2
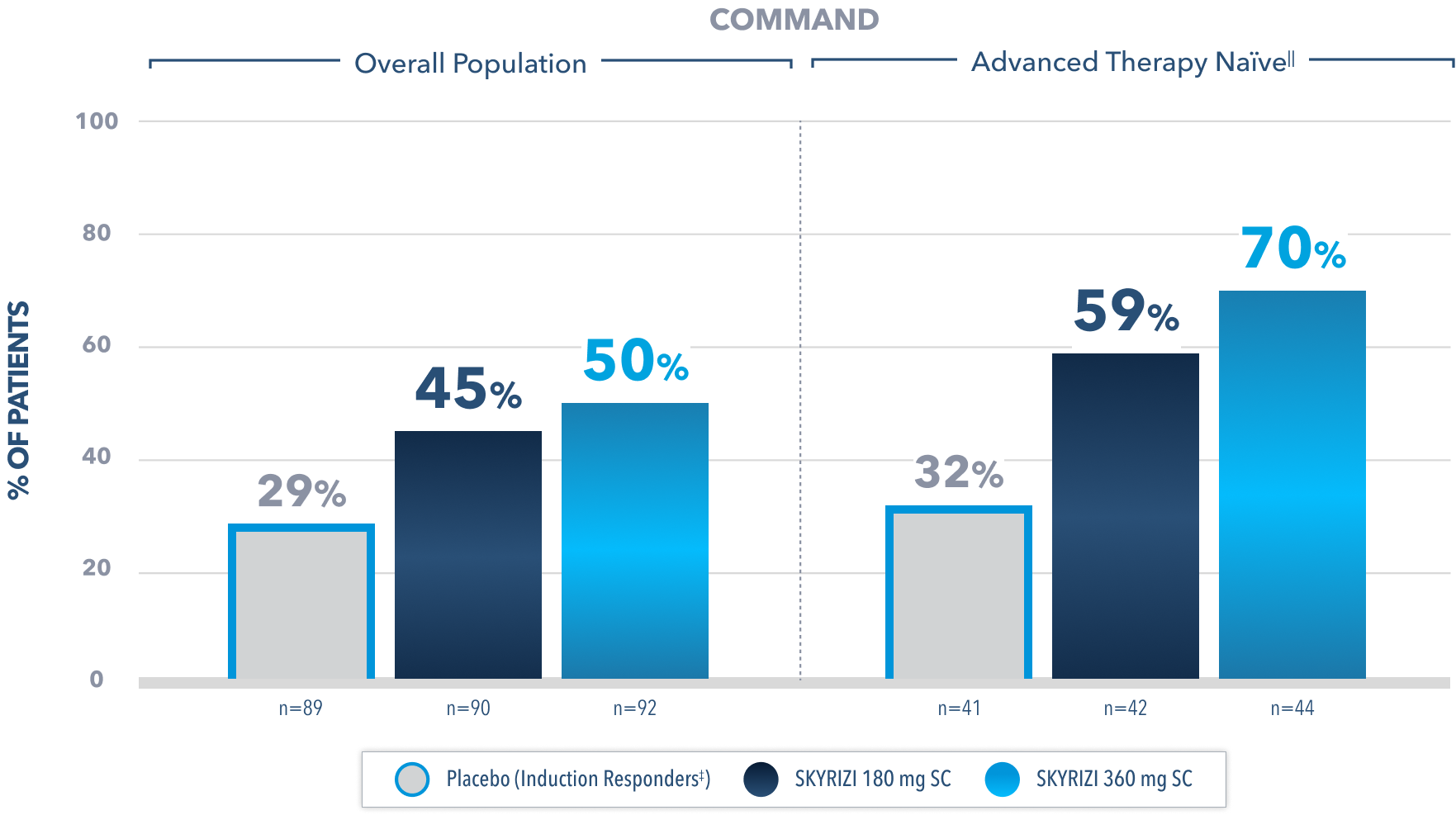
Limitations of prespecified subgroup analysis: Results shown are only among patients who responded to the FDA-approved 1200 mg IV induction dose. Excluded are those who received unapproved induction doses (600 mg or 1800 mg). Data were not controlled for multiplicity. No conclusions or statistical inferences can be made.2
All Subjects¶ Maintenance Population1
At Week 52, proportion of patients who achieved clinical remission* (primary endpoint):
45% SKYRIZI 180 mg SC (n=179; P<0.001)
41% SKYRIZI 360 mg SC (n=186; P<0.001)
26% Placebo (Induction Responders‡) (n=182)
All P-values are SKYRIZI treatment arms vs placebo (induction responders).
¶Patients who responded to 1800 mg, 1200 mg, or 600 mg in Phase 2b/3 induction study.1
*Clinical remission in UC is defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and endoscopic subscore ≤1 without friability.1
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
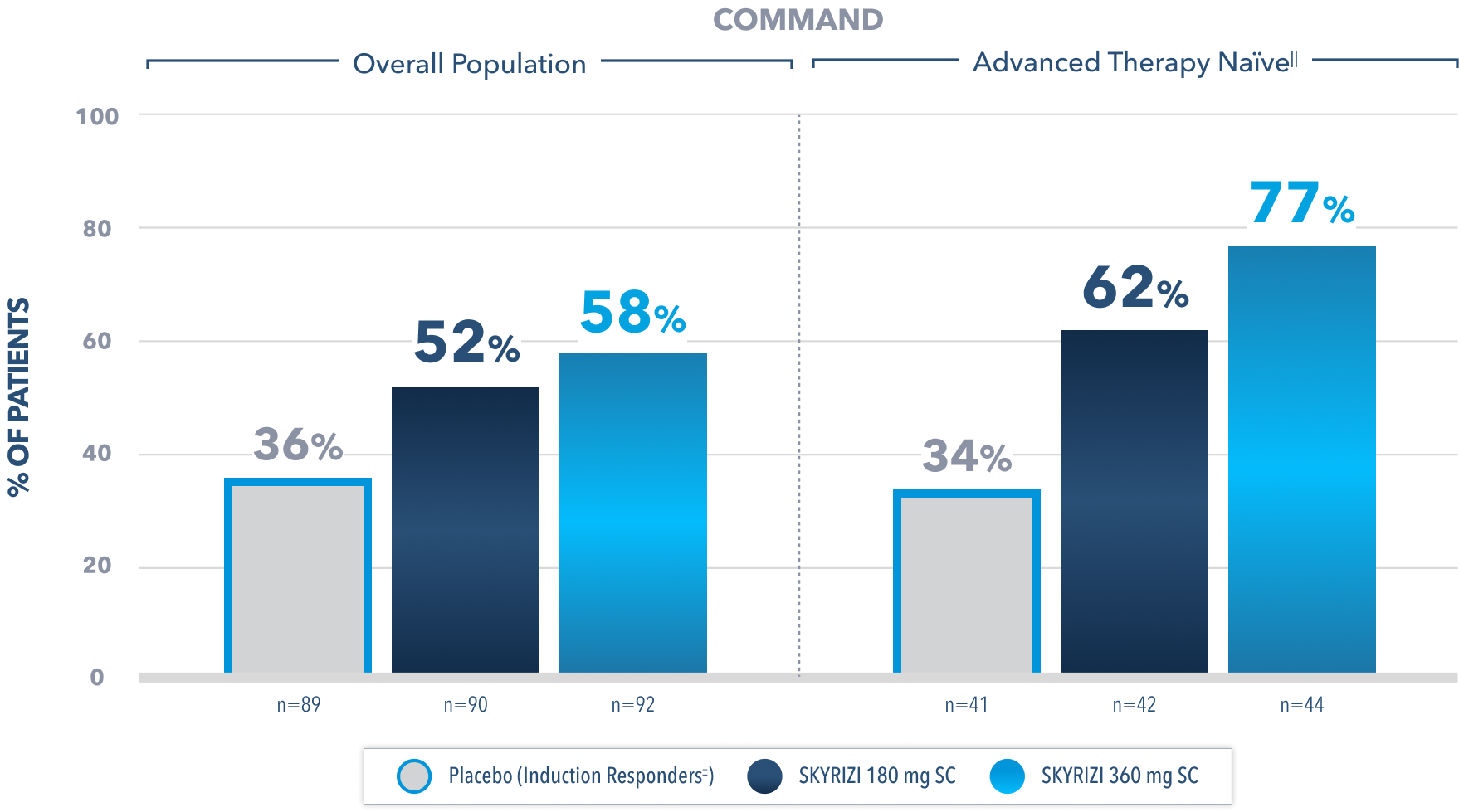
In a post-hoc analysis of patients who responded to the FDA-approved 1200 mg IV induction dose, among those in clinical remission at Week 52,
>97% WERE ALSO CORTICOSTEROID-FREE
for ≥90 days prior to visit2
(180 mg [N=39/40]; 360 mg [N=44/45]; placebo [induction responders] [N=26/26])
Post-Hoc Limitation: Data were not controlled for multiplicity. No conclusions or statistical inferences can be made.
At Week 52, in the prespecified subgroup analysis in patients who responded to the FDA-approved 1200 mg IV induction dose, 44% of patients on SKYRIZI 180 mg and 48% of patients on SKYRIZI 360 mg achieved corticosteroid-free clinical remission compared to 29% on placebo.2
All Subjects¶ Maintenance Population1
At Week 52, proportion of patients who achieved corticosteroid-free clinical remission†† (secondary endpoint):
45% SKYRIZI 180 mg SC (n=179; P<0.001)
40% SKYRIZI 360 mg SC (n=186; P<0.001)
26% Placebo (Induction Responders‡) (n=182)
All P-values are SKYRIZI treatment arms vs placebo (induction responders).
¶Patients who responded to 1800 mg, 1200 mg, or 600 mg in Phase 2b/3 induction study.1
‡Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
††Steroid-free clinical remission in UC is defined as clinical remission per modified Mayo Score (rectal bleeding, stool frequency, endoscopic subscore) at Week 52 and corticosteroid-free for ≥90 days immediately preceding Week 52.1
IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate; SC=subcutaneous.
EARLY DISEASE CONTROL DATA
Clinical Response‡‡ at Weeks 4, 8, 122
(Composite of rectal bleeding and stool frequency subscores)
All P-values are SKYRIZI treatment arms vs placebo.
Data Limitation: Clinical response at Weeks 8 and 12 were prespecified, nonranked endpoints not controlled for multiplicity. No conclusions or statistical inferences can be made.
‡‡Clinical response at Week 4 in UC per partial modified Mayo Score is a composite of Mayo stool frequency and rectal bleeding subscores and was defined as a decrease in total score ≥30% and ≥1 point from baseline and a decrease in rectal bleeding subscore ≥1 or rectal bleeding subscore of 0 or 1.1
IV=intravenous.
No Bowel Urgency§§ at Week 121,2
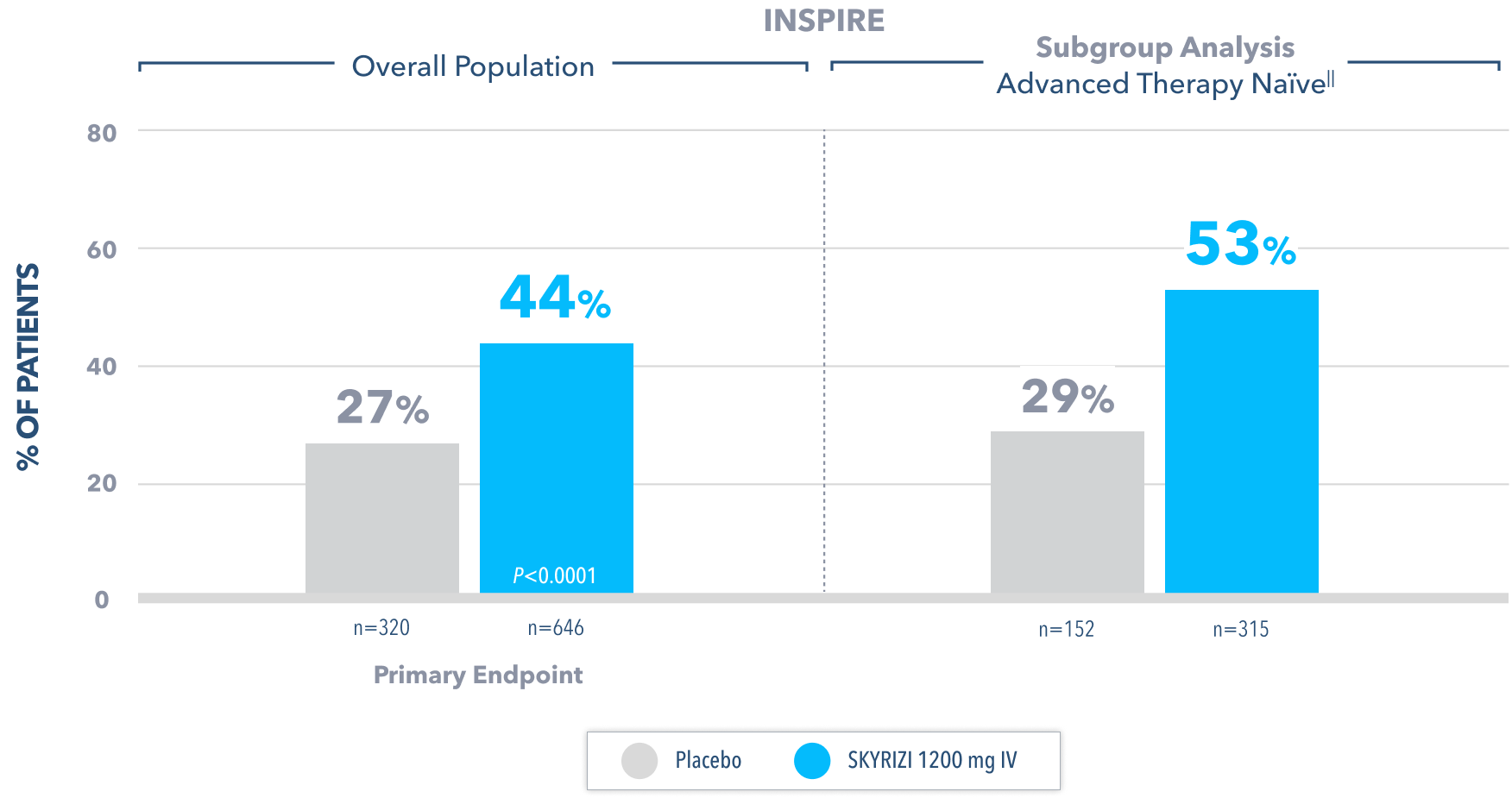
All P-values are SKYRIZI treatment arms vs placebo.
Data Limitation: Bowel urgency at Week 12 in the advanced therapy-naïve subgroup was not controlled for multiplicity. No conclusions or statistical inferences can be made.
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
§§No bowel urgency in UC is defined as if subject reported no bowel urgency on the most recent 3 days up to 10 days prior to each study visit.5
IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate.
Endoscopic Improvement§ at Week 122
(Endoscopy subscore of 0 or 1, without friability)
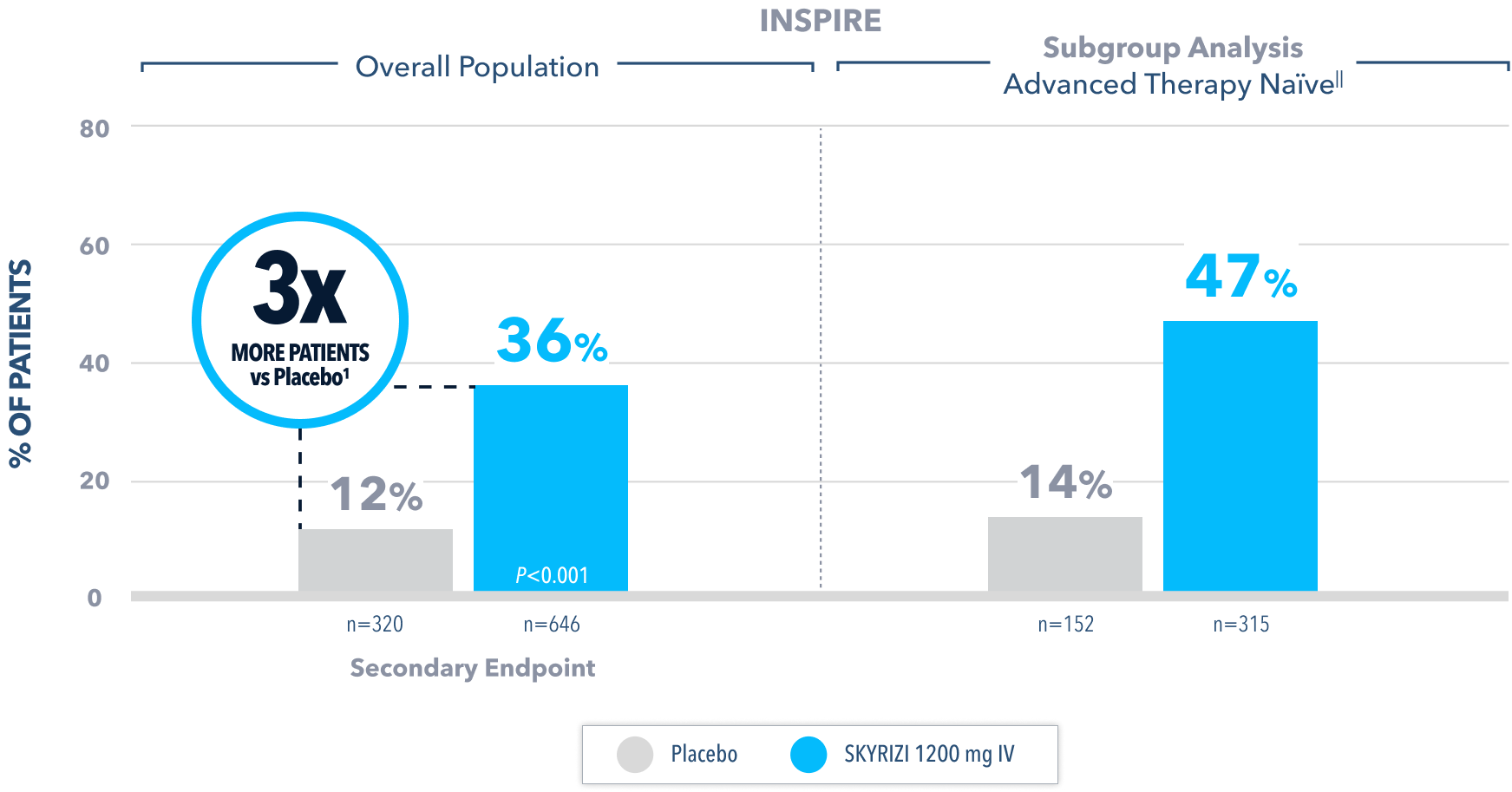
All P-values are SKYRIZI treatment arms vs placebo.
§Endoscopic improvement in UC is defined as Mayo endoscopic subscore of 0 or 1 without friability.1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, at the discretion of the investigator.
IIAdvanced therapy-naïve patients include some patients who were exposed to an advanced therapy (biologics, JAK inhibitors, and/or S1P receptor modulators) but did not experience treatment failure.
IV=intravenous; JAK=Janus kinase; S1P=Sphingosine-1-phosphate.
Fecal Calprotectin Reduction at Weeks 4 and 122,4
Median Fecal Calprotectin2
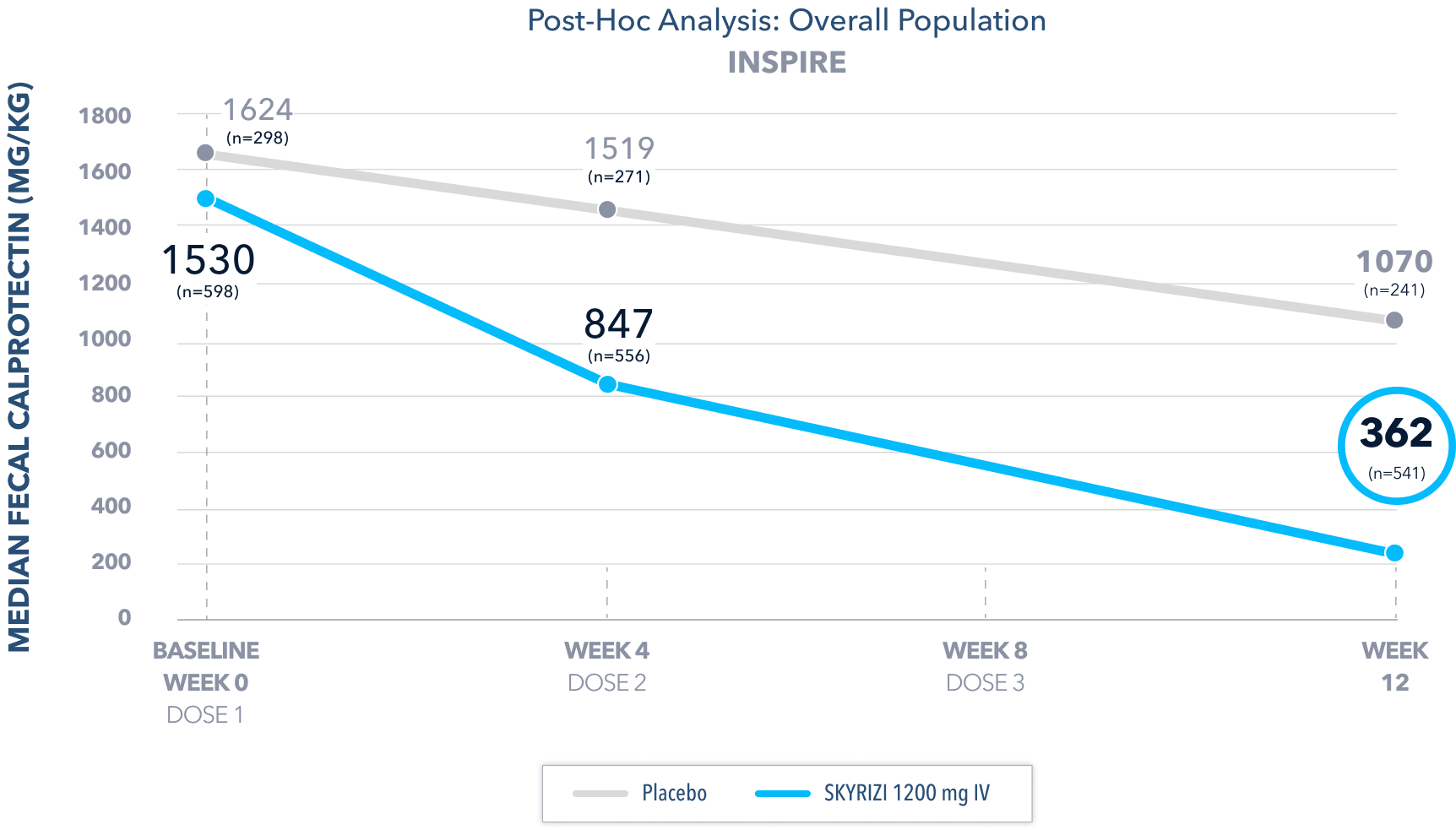
Fecal Calprotectin ≤250 mg/kg in a post-hoc analysis2:
- At Week 4, 25% of patients on SKYRIZI 1200 mg had fecal calprotectin ≤250 mg/kg compared to 9% on placebo
- At Week 12, 40% of patients on SKYRIZI 1200 mg had fecal calprotectin ≤250 mg/kg compared to 15% on placebo
Post-hoc Limitation: Median change from baseline in fecal calprotectin at Week 4 and Week 12 and fecal calprotectin ≤250 mg/kg at Week 4 and Week 12 were post-hoc analyses. Neither of these analyses were adjusted for multiplicity; thus, no statistical inferences can be made.
Fecal calprotectin is not validated as a biomarker to monitor disease progression.
IV=intravenous.
SUPPORT FOR EVERY STAGE OF TREATMENT
SKYRIZI Complete offers your patients support throughout the treatment journey.
RECOMMENDED FOR YOU
SKYRIZI SAFETY
SKYRIZI has a well-studied safety profile.1
SKYRIZI ACCESS & REIMBURSEMENT
AbbVie can help your patients access SKYRIZI.
SKYRIZI DOSING
Learn about SKYRIZI dosing and download a helpful guide.