CONTROL
IS EVERYTHING
CONTROL
IS EVERYTHING
SKYRIZI provides the opportunity for endoscopic and symptom control. For your patients, that’s everything.
SEE EFFICACY DATA FOR ENDOSCOPIC HEALING
Endoscopic improvement,* endoscopic remission,† and histo-endoscopic mucosal improvement (HEMI)‡ at Week 521
The relationship between HEMI to disease progression and long-term outcomes was not evaluated.1
SEE EFFICACY DATA FOR CLINICAL REMISSION
Clinical remission§ and steroid-free clinical remissionll at Week 521
SEE EFFICACY DATA FOR EARLY DISEASE CONTROL
Clinical response¶ as early as Week 41
Clinical remission§ and endoscopic improvement* at Week 121
SEE THE FULL SAFETY PROFILE IN SKYRIZI
Safety profile established across 4 indications with up to ~9 years of clinical trial experience starting in plaque psoriasis1,2
*Endoscopic improvement in UC is defined as Mayo endoscopic subscore of 0 or 1 without friability.1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, at the discretion of the investigator.
†Endoscopic remission in UC is defined as Mayo endoscopic subscore of 0.1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, at the discretion of the investigator.
‡Histo-endoscopic mucosal improvement in UC is defined as Mayo endoscopy subscore of 0 or 1 without friability and Geboes score ≤3.1 (neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue).1 Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, depending on the extent of disease at study entry, and histology results are based on a set of 2 biopsies.
§Clinical remission in UC is defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and endoscopic subscore ≤1 without friability.1
llSteroid-free clinical remission in UC is defined as clinical remission per modified Mayo Score (rectal bleeding, stool frequency, endoscopic subscore) at Week 52 and corticosteroid-free for ≥90 days immediately preceding Week 52.1
¶Clinical response at Week 4 in UC per partial modified Mayo Score is a composite of Mayo stool frequency and rectal bleeding subscores and was defined as a decrease in total score ≥30% and ≥1 point from baseline and a decrease in rectal bleeding subscore ≥1 or rectal bleeding subscore of 0 or 1.1
IL-23i=interleukin-23 inhibitor.
SKYRIZI MET ITS PRIMARY ENDPOINTS1
INSPIRE PHASE 3 INDUCTION
Clinical Remission§ at Week 12:
8% Placebo (n=320)
COMMAND ALL SUBJECTS MAINTENANCE POPULATION#
Clinical Remission§ at Week 52:
26% Placebo (Induction Responders**) (n=182)
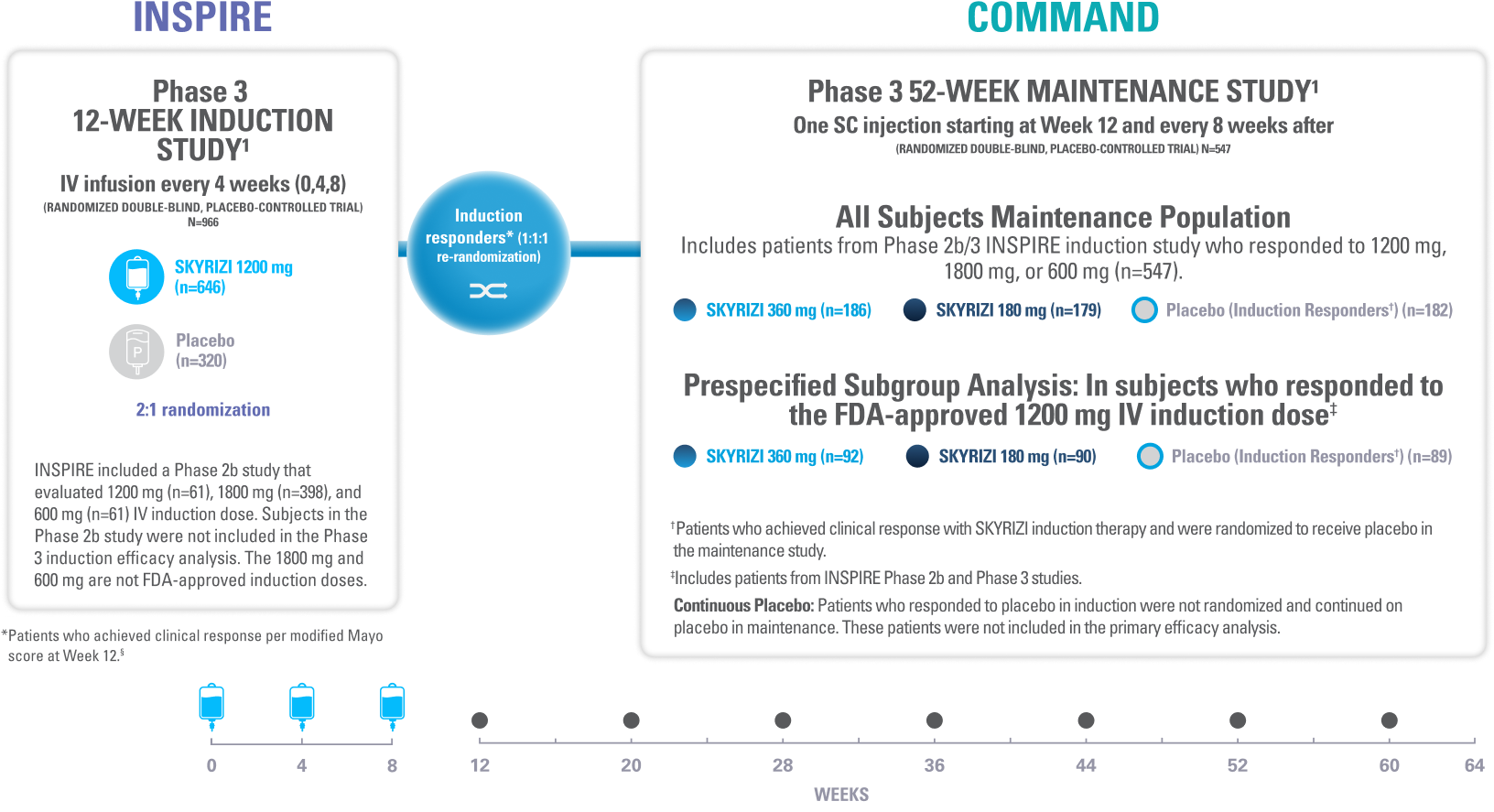
#Includes patients who responded to 1800 mg, 1200 mg, or 600 mg in the Phase 2b/3 induction study; 600 mg and 1800 mg are not FDA-approved induction doses.1
All P-values are SKYRIZI treatment arms vs placebo.
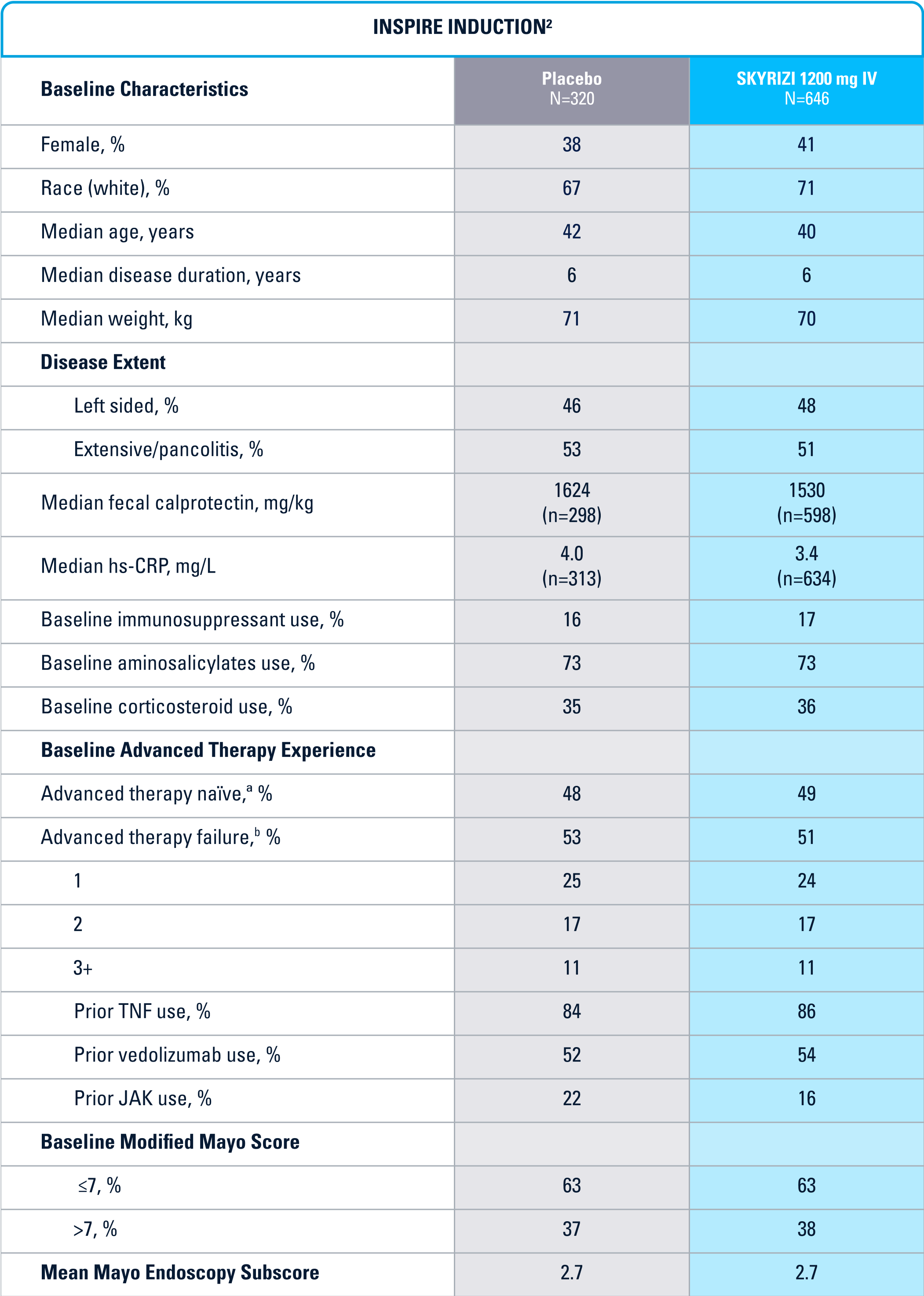
INSPIRE Induction (N=966) was a Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SKYRIZI 1200 mg IV, at Weeks 0, 4, and 8 vs placebo over 12 weeks in adult patients with moderate to severe UC. The primary endpoint was clinical remission§ at Week 12.1
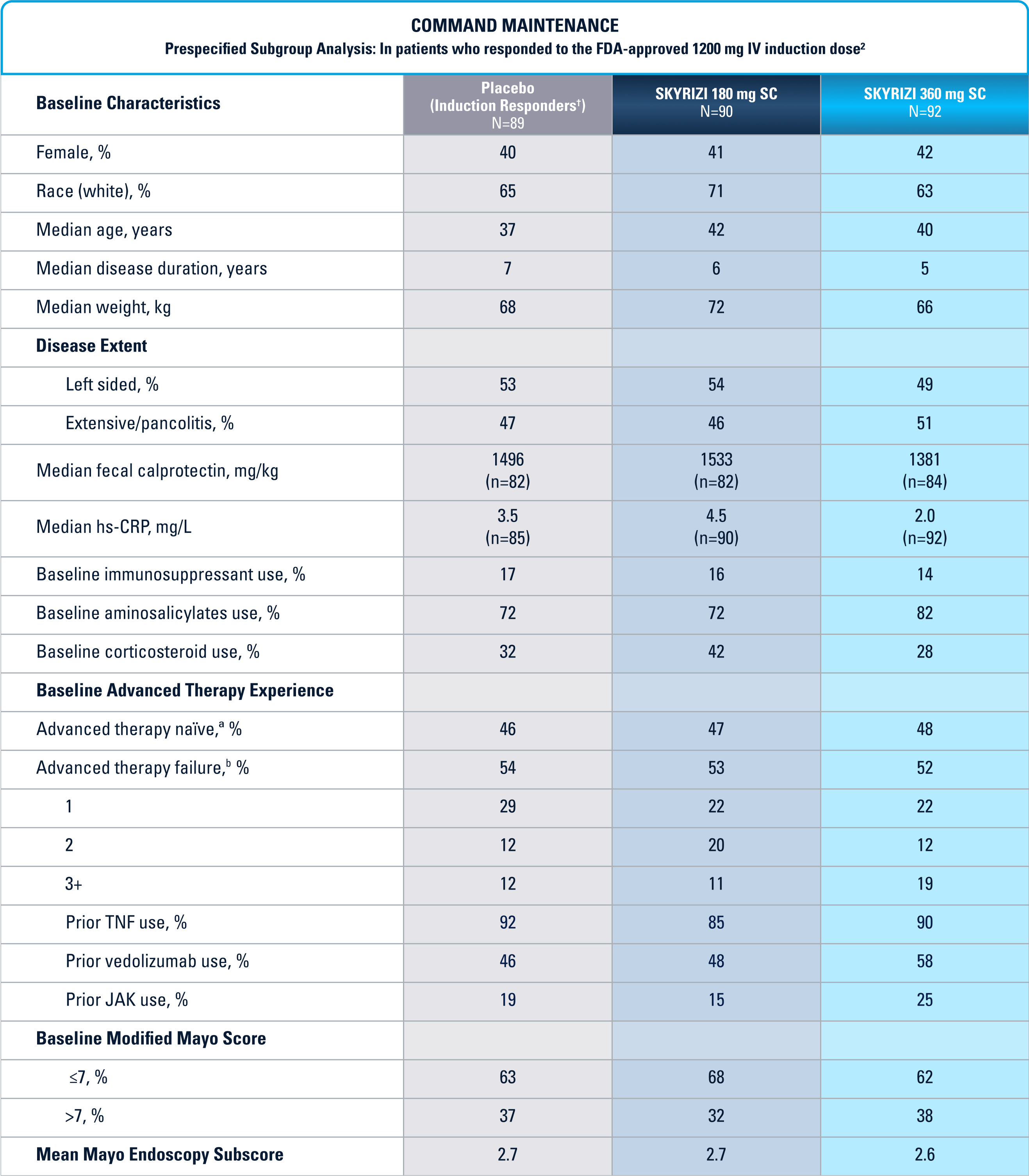
COMMAND Maintenance (N=547) was a Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SKYRIZI 180 mg or 360 mg SC vs placebo up to 52 weeks in adult patients who were clinical responders to one of three SKYRIZI induction regimens consisting of 600 mg, 1200 mg or 1800 mg IV (the 600 mg and 1800 mg IV are not FDA-approved induction regimens) in the INSPIRE Phase 2b or Phase 3 studies. The primary endpoint was clinical remission§ at Week 52. COMMAND also included a prespecified subgroup analysis inclusive only of patients who responded to the FDA-approved induction dosing of SKYRIZI 1200 mg IV (N=271).1
§Clinical remission in UC is defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and endoscopic subscore ≤1 without friability.1
**Patients who achieved clinical response with SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.1
IV=intravenous; SC=subcutaneous.
SKYRIZI COMPLETE SUPPORT PROGRAM
Providing support for your patients throughout the treatment journey.
Help patients start and stay on track with their SKYRIZI treatment plan.
Download a guide that includes relevant codes and helpful tips.