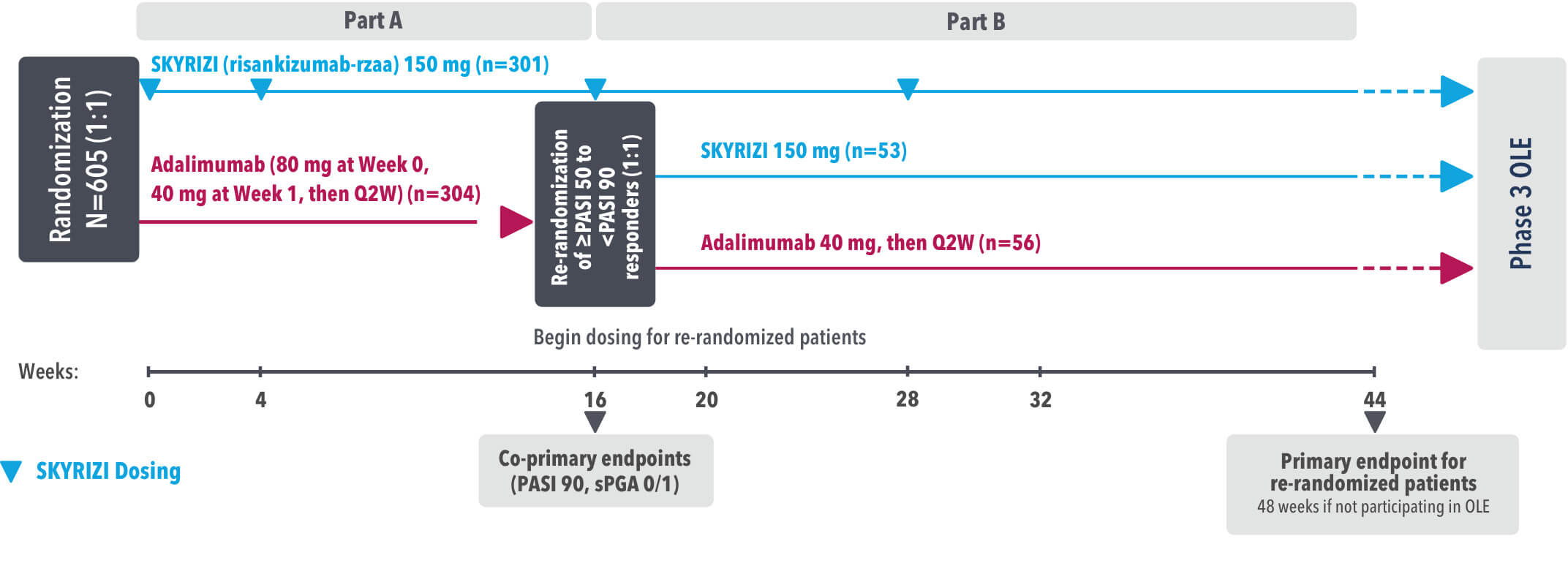
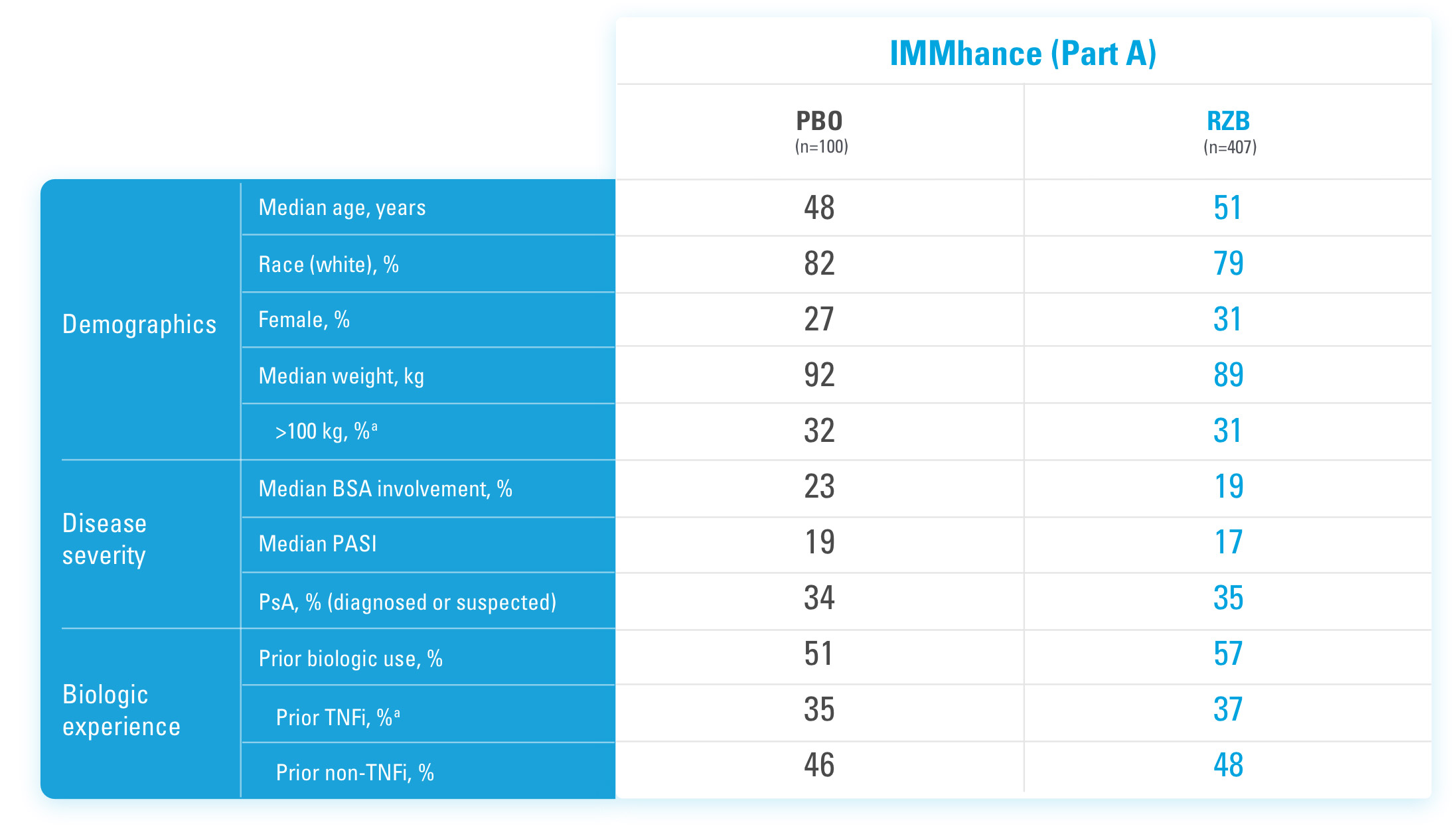
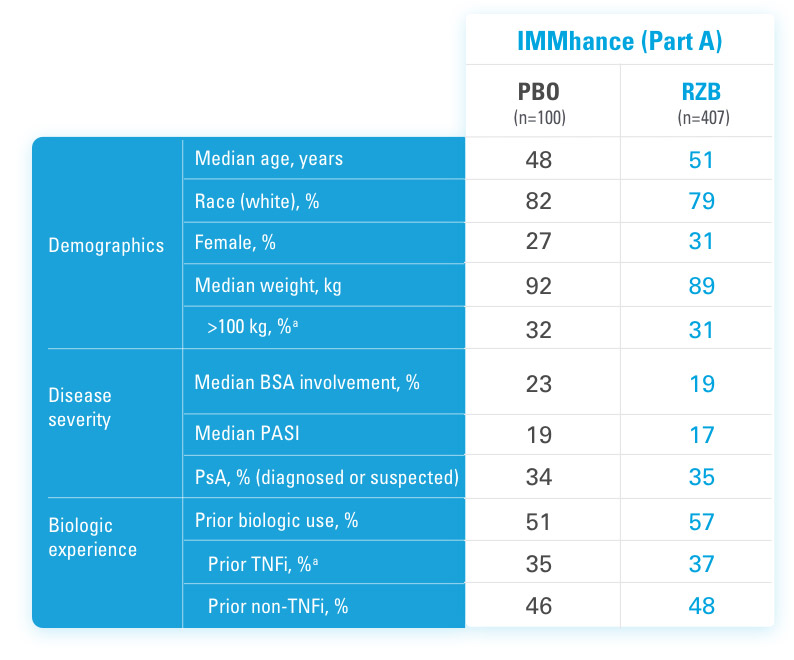
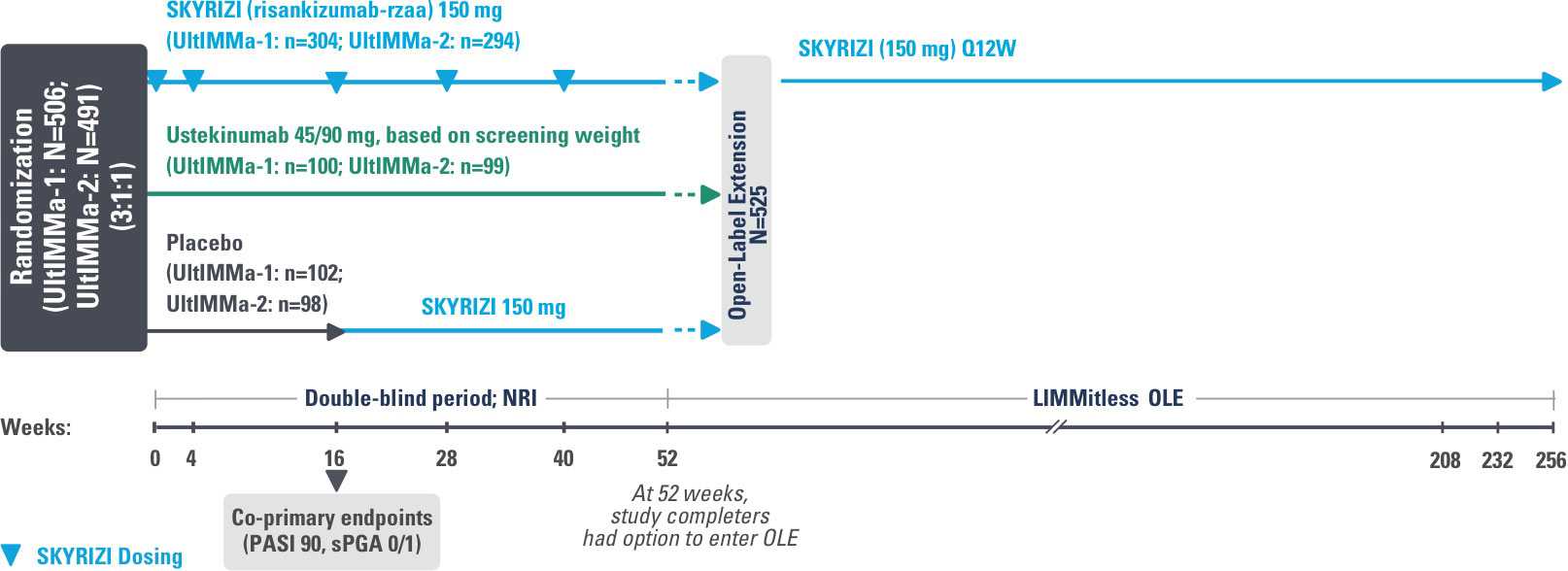
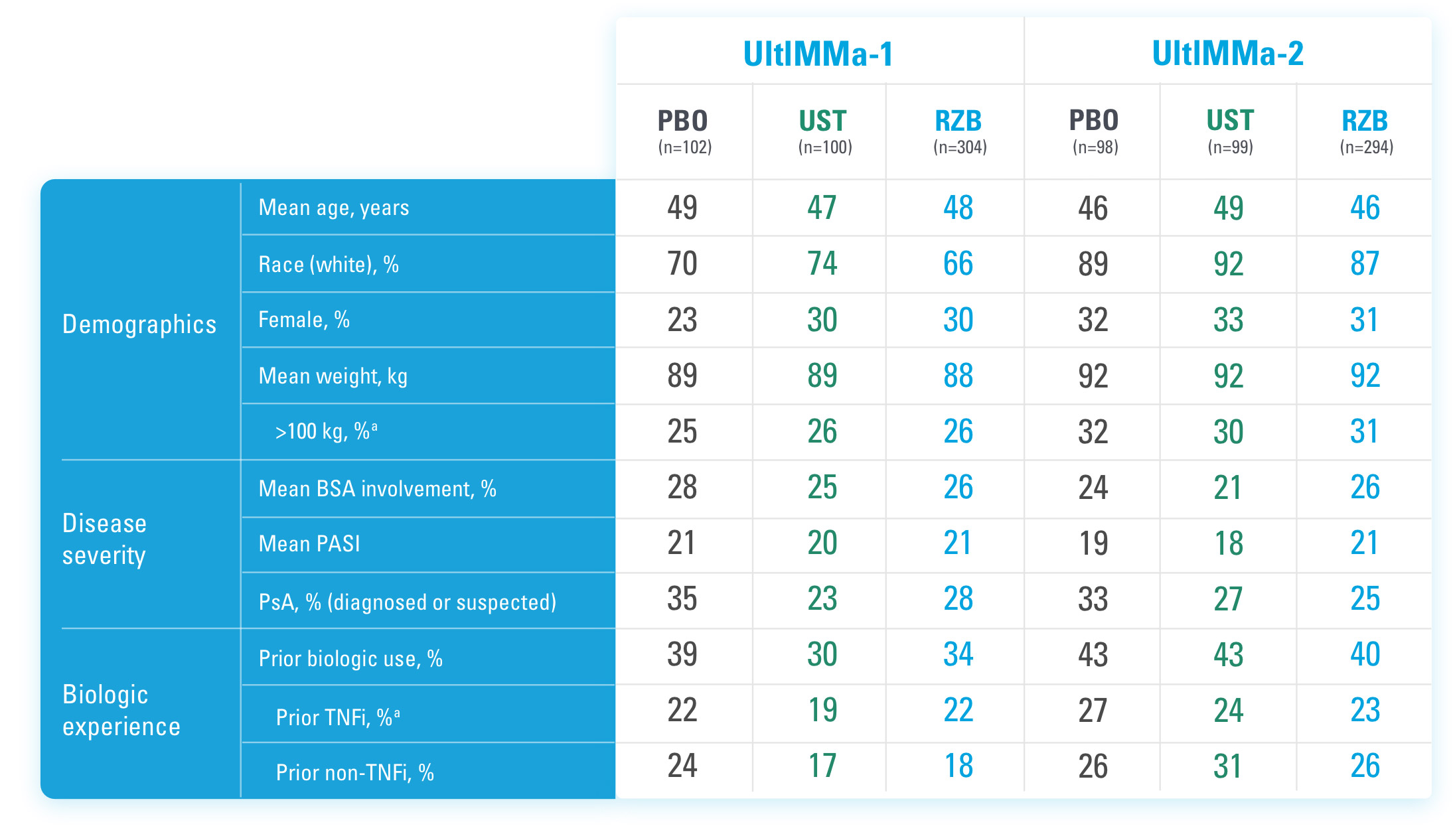
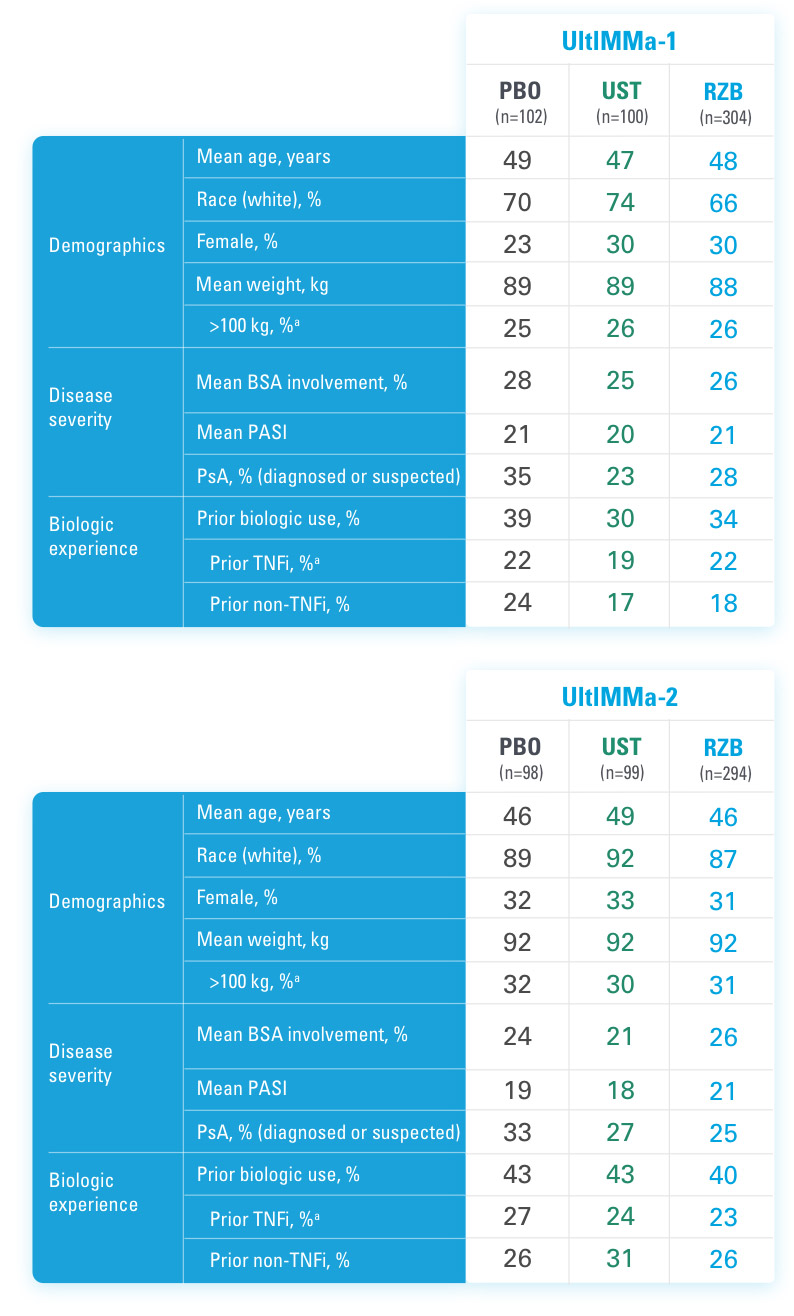
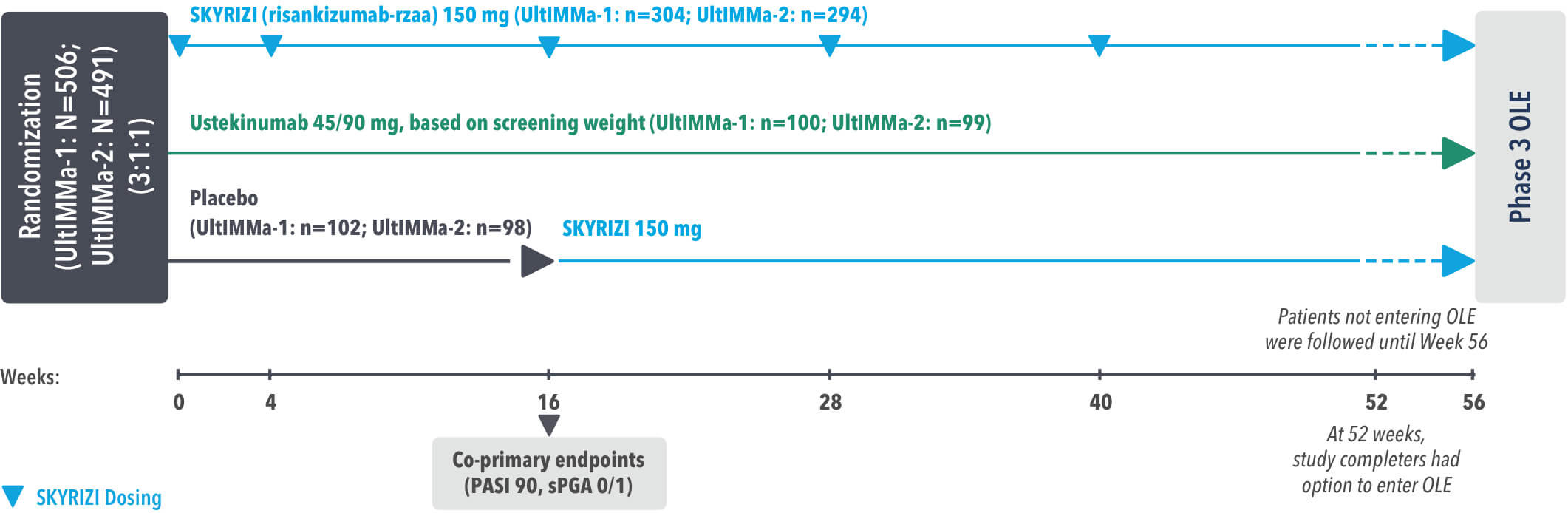
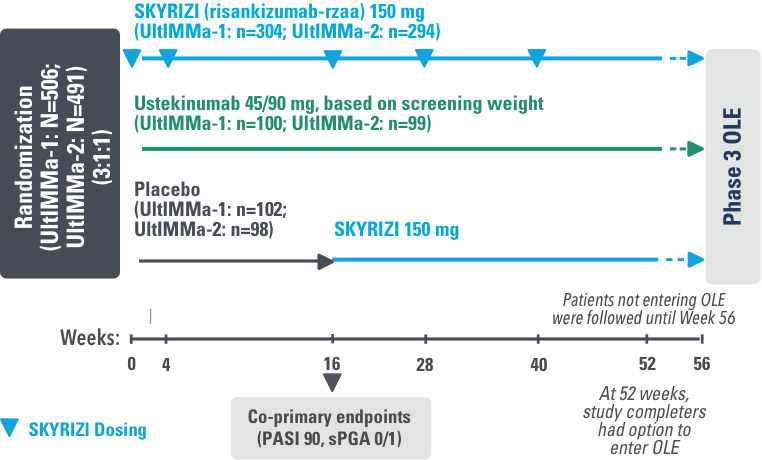
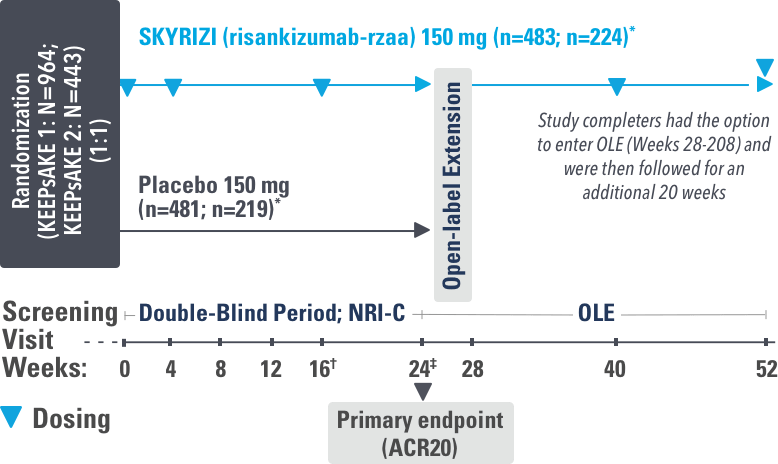
INJECTING WITH THE SKYRIZI PEN
DISCLAIMER
Super: This demonstration is a guide to injecting with the SKYRIZI Pen. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject. Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
Audio: This demonstration is a guide to injecting with the SKYRIZI Pen. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject.
Super: SKYRIZI® (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
- with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
- with active psoriatic arthritis (PsA).
Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
Audio: SKYRIZI (risankizumab-rzaa) uses
SKYRIZI is a prescription medicine used to treat adults: with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).with active psoriatic arthritis (PsA).
Super: SAFETY CONSIDERATIONS
SKYRIZI may cause serious side effects, including:
- Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
- Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI.
Also, tell your doctor if you plan to or recently received a vaccine.
Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
Audio: Safety considerations.
SKYRIZI may cause serious side effects, including:
Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. Also, tell your doctor if you plan to or recently received a vaccine.
Video: Cut to table shot with Sharps Container and Pen package.
Super: A GUIDE TO INJECTING WITH SKYRIZI
Audio: Hi. I’m Michael.
Video: Cut to Michael facing camera.
Super: MICHAEL
Audio: And I’m here to show you how to inject using a SKYRIZI Pen.
Video: Cut to wider shot of Michael.
Audio: I’ve been doing this for awhile now, and I feel like I have a good handle on it. Ah, my doctor showed me how to inject when I first started.
Video: Cut to phone with Nurse Ambassador screen displayed
Super: Don’t have a Nurse Ambassador?* Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
SKYRIZI logo
Audio: And I called Lauren, my Nurse Ambassador, and . . .
Video: Michael sitting at table.
Super: Don’t have a Nurse Ambassador?* Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
Audio: . . . she helped me the first few times I did it at home by myself.
Video: Cut to table shot of alcohol pad, Pen package, Sharps Container, and instructions.
Super: INJECTION PREP
Audio: Now, I get my SKYRIZI by mail . . .
Video: Cut to Michael opening the refrigerator
Audio: . . . and I keep it in the fridge until I’m ready to inject.
Video: Shot continues, Michael reaches into refrigerator.
Audio: Ah, there it is.
Super: Shot continues, Michael closes refrigerator.
Audio: There we go.
Video: Shot continues, Michael puts SKYRIZI package on table
Super: Do not warm SKYRIZI in any other way (for example, do not warm it in a microwave or in hot water).
Audio: So, very important.
Video: Shot continues as Michael continues talking.
Super: Do not use SKYRIZI if liquid has been frozen or even if it has been thawed.
I’ll leave it out at room temperature and out of direct sunlight for 30 to 90 minutes before injecting.
Video: Shot continues, Michael walks off screen.
Super: 30 to 90 minutes
Keep SKYRIZI in the original carton to protect from light until time to use.
Do not use SKYRIZI if liquid has been frozen or even if it has been thawed.
Video: Cut to Michael sitting at table
Super: Do not shake SKYRIZI.
Audio: Now that I’ve washed and dried my hands, I’m good to go.
Video: Shot continues, Michael continues talking.
Super: Do not use if the SKYRIZI Pen has been dropped or damaged.
Audio: So, I take out everything I need. Ah, I got my SKYRIZI Pen.
Video: Cut to table shot with phone, Pen, package, instructions, alcohol swab, Sharps Container, and cotton ball. Michael points at each.
Super: SKYRIZI PEN
Do not use if the SKYRIZI Pen has been dropped or damaged.
Video: Shot continues, Michael continues talking and pointing at items.
Super: SKYRIZI PEN
INSTRUCTIONS FOR USE
ALCOHOL SWAB
not included in your package
Do not use SKYRIZI if package perforations are broken. Return product to the pharmacy.
Audio: I’ve got the Instructions for Use for reference. I’ve also got an alcohol swab . . .
Video: Shot continues, Michael continues talking and pointing at items
Super: SKYRIZI PEN
INSTRUCTIONS FOR USE
ALCOHOL SWAB
not included in your package
COTTON BALL
not included in your package
SHARPS CONTAINER
Do not remove the dark gray cap until right before injection.
Audio: . . . a cotton ball, and my sharps container. So . . .
Video: Cut to Michael sitting at table
Super: Do not remove the dark gray cap until right before injection.
Audio: . . . I do my injections . . .
Video: Cut to Michael sitting at table.
Super: Do not use SKYRIZI if expiration date (EXP) has passed.
Audio: . . . here because there’s just plenty of light and a lot of good space.
Video: Cut to table shot with alcohol swab, package with Pen, Sharps Container, and instructions.
Super: HOW TO INJECT
step-by-step
Audio: I like to think about the injection process like this . . .
Video: Cut to wide shot of inside of package.
Audio: Pick. Pull. Place. Push. And press. So first . . .
Video: Cut to wide shot of Michael indicating his thigh.
Super: PICK
the injection site
Audio: . . . I’ll pick my injection site. I’m gonna go with my left thigh. But you can also inject into your stomach or your other thigh.
Video: Shot continues, copy fades off screen, Michael indicates his stomach.
Audio: If you choose your stomach, make sure that you inject . . .
Video: Shot continues, Michael continues talking and indicating his stomach.
Super: 2 INCHES
Square graphic with arrows.
Audio: ...at least two inches away from your belly button.
Video: Shot continues, copy and graphic fade off screen, Michael continues talking.
Audio: Grab your alcohol swab and clean your skin in . . .
Video: Cut to close-up of Michael’s thigh. He rubs it with the alcohol swab.
Super: Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
Audio: . . . a circular motion like this. And let it dry.
Video: Cut to shot of Michael at table.
Super: Do not inject through clothes.
Audio: Now, I hold the Pen with the dark gray cap pointing up.
Video: Shot continues as Michael pulls the cap off of the Pen and continues talking.
Super: PULL
the cap off
Do not inject into skin that is sore, bruised, red, hard, scarred, has stretch marks, or areas with psoriasis.
Audio: I pull the cap straight off and throw it away. Now . . .
Video: Cut to close-up of Michael holding the pen.
Audio: . . . I check the liquid through the inspection window.
Video: Shot continues as Michael continues talking.
Super: Do not use if the liquid is cloudy or contains flakes or large particles.
Audio: It should look clear to slightly yellow and may contain tiny white or clear particles. It’s normal to see one or more bubbles in the liquid.
Video: Cut to wide shot of Michael holding Pen horizontally.
Audio: So, I hold my Pen with my fingers on the gray grips. See?
Video: Shot continues as Michael turns the Pen and holds it vertically, indicating the activator button. Inset shot shows close up of needle sleeve.
Super: NEEDLE SLEEVE
Audio: And I turn the Pen so that the white needle sleeve points toward my injection site and I can see the green activator button.
Video: Cut to close-up of Pen showing injection window and needle sleeve.
Audio: I also make sure that I can clearly see the inspection window while I’m injecting.
Video: Cut to Michael lowering the Pen to his thigh and squeezing the skin at the injection site.
Audio: I gently squeeze my skin at my injection site and make a raised area and hold it firmly. Then . . .
Video: Shot continues as Michael continues talking and lowering the Pen to his thigh.
Super: PLACE
Audio: . . . I place the white needle sleeve straight . . .
Video: Cut to close-up of Michael holding the Pen against his thigh.
Super: PLACE & PUSH
against skin
90°
Right angle graphic
Audio: . . . against the raised area, at a 90 degree angle, and push the Pen down against my skin. Throughout the process, I keep pinching . . .
Video: Close-up of Michael injecting into his thigh.
Super: PLACE & PUSH
against skin
Audio: . . . the raised area and keep steady pressure against it
Video: Cut to different angle of Michael injecting.
Audio: Now I can see the green activator button and inspection window.
Video: Cut to split screen of Michael injecting.
Super: PUSH
against skin
The Pen will activate only if the white needle sleeve is firmly pushed down against the injection site before pressing the green activator button.
Audio: The Pen only activates if the white needle sleeve is pressed firmly down against me before pressing the green activator button. A loud . . .
Video: Cut to close up of Michael holding Pen against his thigh.
Super: PRESS
the green button and keep steady pressure
Audio: . . . click means the start of the injection.
Video: Clock graphic animates on and counts down from 00:15 seconds.
Super: PRESS
the green button and keep steady pressure
Audio: SFX: Click sound
There it goes. And hold it like that for 15 seconds.
Video: Shot continues as copy fades off screen.
Graphic on screen:
Clock graphic counting down
Audio: Now, I’ll keep pushing the Pen down against my skin until I hear a second click . . . or the yellow indicator has filled the inspection window—either one signals the injection is complete.
Video: Shot continues as graphics fade off screen and inset of injection window appears.
Audio: SFX: Click sound
And there it goes. I check to make sure that the yellow indicator is fully down to be sure the injection is complete. Only then do . . .
Video: Shot continues as Michael lifts the Pen from his thigh.
Audio: . . . I slowly pull the pen straight out from my skin.
SFX: Click sound
You might notice one last click.
Video: Cut to close-up of Michael indicating the white needle sleeve of the Pen.
Audio: And look at this. You see how the white needle sleeve covers the needle tip?
Video: Cut to wide shot of Michael holding the pen.
Audio: And that’s it.
Video: Shot continues as copy animates on screen.
Super: Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
Audio: Now, I press a cotton ball where I injected. You could also use a gauze pad.
Video: Shot continues as copy animates off screen and new copy fades in.
Super: Do not rub the injection site. You may have slight bleeding. This is normal.
Audio: And I drop my used Pen . . .
Video: Cut to close-up of Michael placing Pen in Sharps Container.
Super: Do not rub the injection site. You may have slight bleeding. This is normal.
Audio: . . . in one of these Sharps Disposal Containers.
Video: Michael carries the Sharps Container and places it on a high shelf.
Super: Your Skyrizi Complete Sharps Disposal Container may look a little different than the one shown.
Audio: There. Safe and sound and also out of reach.
Video: Cut to shot of Michael sitting at table.
Audio: Another injection complete.
Video: Cut to table shot with alcohol swab, package with Pen, Sharps Container, and instructions.
Super: HELPFUL TIPS
for self-injecting
Audio: To recap . . .
Video: Cut to wide shot of Michael sitting at table.
Audio: . . . I prepare myself and my space.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
Audio: Pick an injection site.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
PULL the cap off
Audio: Pull the cap off the Pen.
Video: Shot continues as screens with copy animate in
Super: PICK the injection site
PULL the cap off
PLACE Pen on site
Audio: Place the Pen on the injection site.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
PULL the cap off
PLACE Pen on site
PUSH firmly down against skin
Audio: Push the Pen firmly down against my skin.
Video: Shot continues as screens with copy animate in.
Super: PULL the cap off
PLACE Pen on site
PUSH firmly down against skin
PRESS the button
Audio: Press the button to inject. And keep steady pressure on the injection site for the full 15 seconds.
Video: Cut to close-up of Michael placing the lid on the Sharps Container.
Audio: Then safely discard the used Pen.
Video: Shot continues as Michael’s hands leave the frame.
Super: DISCARD used Pen
Audio: That’s the whole injection process.
Video: Cut to medium shot of Michael at table.
Audio: When I started injecting at home, it didn’t take me long to get it down. Lauren, my Nurse Ambassador, she suggested I create a routine. Inject at the same time and place.
Video: Cut to wide shot of Michael at table.
Audio: I also like to treat myself after each injection. And maybe I’ll cycle or listen to a new album.
Video: Cut to table shot. Michael indicates phone screen showing Skyrizi Complete App.
Audio: And this Skyrizi Complete App helps me stay on track, and I don’t know what I’d do without it.
Video: Cut to medium shot of Michael at table.
Audio: I used it to log my first starter dose, so I knew when to take my second starter dose four weeks later. And you can . . .
Video: Cut to table shot. Michael indicates phone screen showing a different screen of the Skyrizi Complete App.
Audio: . . . set reminders for future doses, too, since after two starter doses, . . .
Video: Cut to medium shot of Michael sitting at table.
Super: 1 INJECTION 4x A YEAR
Audio: . . . SKYRIZI is only 1 injection, 4 times a year.
Video: Cut to table shot showing phone with reminder message.
Audio: And when I get those reminders . . .
SFX: Ping sound
I know to contact my specialty pharmacy to get my next injection on time.
Video: Cut to wide shot of Michael at table.
Super: Get sharps containers at no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
Audio: And don’t forget about the Skyrizi Complete Sharps Disposal and mail-back service.
Video: Cut to table shot with alcohol swab and Michael putting lid on Sharps Container.
Super: Get sharps containers at no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
Audio: I signed up online, so once my Sharps Container gets full, . . .
Video: Cut to wide shot of Michael sitting at the table.
Audio: . . . I request a new one. Which is sent to me with a box to send my full one back. It’s so convenient, and it doesn’t cost me anything.
Video: Cut to medium shot of Michael sitting at the table.
Audio: And I’m lucky that my family’s always around if I need help.
Video: Shot continues, copy fades on screen.
Super: CALL 1.866.SKYRIZI
FOR 24/7 SUPPORT WITH
SKYRIZI COMPLETE
Audio: . . . and a pretty awesome app.
Video: Cut to wide shot of Michael sitting at table.
Audio: You’ve got this.
Video: Cut to USES and ISI screen. Copy on screen scrolls:
Super:
USES AND IMPORTANT SAFETY INFORMATION1
SKYRIZI® (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
- with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
- with active psoriatic arthritis (PsA).
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SKYRIZI® (risankizumab-rzaa)?
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
USES AND IMPORTANT SAFETY INFORMATION
SKYRIZI (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
with active psoriatic arthritis (PsA).
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SKYRIZI (risankizumab-rzaa)?
Video: Scroll continues.
Super: SKYRIZI is a prescription medicine that may cause serious side effects, including:
Serious allergic reactions:
- Stop using SKYRIZI and get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
SKYRIZI is a prescription medicine that may cause serious side effects, including:
Serious allergic reactions:
- Stop using SKYRIZI and get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
Video: Scroll continues.
Super:
Infections:
SKYRIZI may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with SKYRIZI and may treat you for TB before you begin treatment with SKYRIZI if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with SKYRIZI.
- Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
Infections
SKYRIZI may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with SKYRIZI and may treat you for TB before you begin treatment with SKYRIZI if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with SKYRIZI.
- Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
Video: Scroll continues.
Super:
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
Before using SKYRIZI, tell your healthcare provider about all of your medical conditions, including if you:
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
Before using SKYRIZI, tell your healthcare provider about all of your medical conditions, including if you:
Video: Scroll continues.
Super:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about SKYRIZI?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). Medications that interact with the immune system may increase your risk of getting an infection after receiving live vaccines. You should avoid receiving live vaccines right before, during, or right after treatment with SKYRIZI. Tell your healthcare provider that you are taking SKYRIZI before receiving a vaccine.
- are pregnant or plan to become pregnant. It is not known if SKYRIZI can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SKYRIZI passes into your breast milk.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about SKYRIZI?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). Medications that interact with the immune system may increase your risk of getting an infection after receiving live vaccines. You should avoid receiving live vaccines right before, during, or right after treatment with SKYRIZI. Tell your healthcare provider that you are taking SKYRIZI before receiving a vaccine.
- are pregnant or plan to become pregnant. It is not known if SKYRIZI can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SKYRIZI passes into your breast milk.
Video: Scroll continues.
Super:
- become pregnant while taking SKYRIZI. You are encouraged to enroll in the Pregnancy Registry, which is used to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-302-2161 to enroll in this registry.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of SKYRIZI?
SKYRIZI may cause serious side effects. See “What is the most important information I should know about SKYRIZI?”
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
- become pregnant while taking SKYRIZI. You are encouraged to enroll in the Pregnancy Registry, which is used to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-302-2161 to enroll in this registry.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of SKYRIZI?
SKYRIZI may cause serious side effects. See “What is the most important information I should know about SKYRIZI?”
Video: Scroll continues.
Super:
The most common side effects of SKYRIZI in people treated for plaque psoriasis and psoriatic arthritis include: upper respiratory infections, headache, feeling tired, injection site reactions, and fungal skin infections.
These are not all the possible side effects of SKYRIZI. Call your doctor for medical advice about side effects.
Use SKYRIZI exactly as your healthcare provider tells you to use it.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI
SKYRIZI logo.
Audio:
The most common side effects of SKYRIZI in people treated for plaque psoriasis and psoriatic arthritis include: upper respiratory infections, headache, feeling tired, injection site reactions, and fungal skin infections.
These are not all the possible side effects of SKYRIZI. Call your doctor for medical advice about side effects.
Use SKYRIZI exactly as your healthcare provider tells you to use it.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Video: Scroll continues.
Super:
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/myAbbVieAssist to learn more.
Reference: 1. SKYRIZI [package insert]. North Chicago, IL. AbbVie Inc.
US-SKZD-220756
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
If you are having difficulty paying for your medicine, AbbVie may be able to help.
Visit AbbVie.com/myAbbVieAssist to learn more.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI