AT WEEK 16
Placebo 30% (n=81)
US-MULT-240253
The IL-23 inhibitor from AbbVie indicated for the treatment of
moderate to severe plaque psoriasis (Ps) in adults who are candidates for systemic
therapy or phototherapy and for adults with active psoriatic arthritis (PsA).1
Challenging areas of the body
SCALP, PALMOPLANTAR, AND NAIL PSORIASIS
PASI 90 at Week 16
UltIMMa-1:
SKYRIZI 75% (229/304), placebo 5% (5/102)
UltIMMa-2:
SKYRIZI 75% (220/294), placebo 2% (2/98)
p<0.0001.
sPGA 0/1 at Week 16
UltIMMa-1:
SKYRIZI 88% (267/304), placebo 8% (8/102)
UltIMMa-2:
SKYRIZI 84% (246/294), placebo 5% (5/98)
NRI=Non-responder imputation.
UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. Patients received SKYRIZI 150 mg at Week 0, Week 4, and every 12 weeks thereafter.1,4
IMMprint, a randomized, double-blind,
placebo-controlled, Phase 3b study
Evaluating SKYRIZI in moderate to severe Ps
patients with palmoplantar involvement
PROPORTION OF PATIENTS ACHIEVING ppIGA 0/1 (NRI-C)
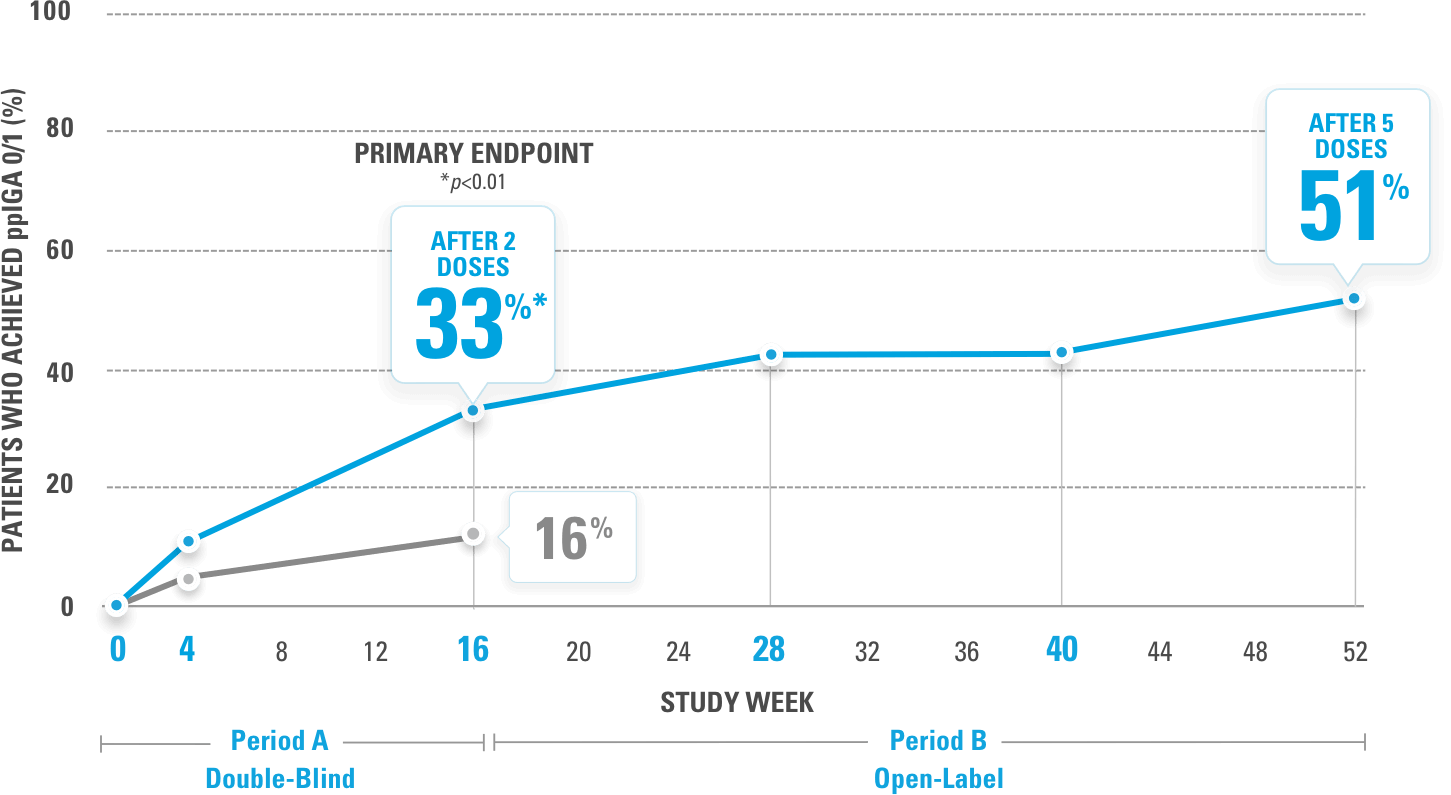
The primary endpoint was ppIGA 0/1 at Week 16.
LIMITATIONS:
All endpoints at Week 52 were pre-specified, non-ranked endpoints and were not controlled for multiple comparisons. Therefore, no statistical or clinical conclusions can be drawn.
Open-Label Limitation:
In an open-label study or period, there is potential for enrichment; awareness of active treatment may cause bias to overall treatment effect.
The ppIGA scale ranges from clear (0) to severe (4) and is based on the modified IGA but specifically applied to the palms and soles.
STUDY DESIGN:
IMMprint was a Phase 3b, multicenter, randomized, double-blind, parallel-group, placebo-controlled, 52-week study that assessed the safety and efficacy of SKYRIZI (150 mg) vs placebo for the treatment of moderate to severe plaque psoriasis in patients with palmoplantar (non-pustular) involvement. The primary endpoint evaluated the proportion achieving ppIGA of 0 or 1 with ≥2-point reduction from baseline at Week 16. Additional ranked secondary endpoints included PPASI 75, PPASI 90, sPGA 0/1, and PPASI 100 at Week 16.2
NRI-C=non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PPASI=Palmoplantar Psoriasis Area and Severity Index; ppIGA=Palmoplantar Psoriasis Investigator's Global Assessment; sPGA=static Physician's Global Assessment.
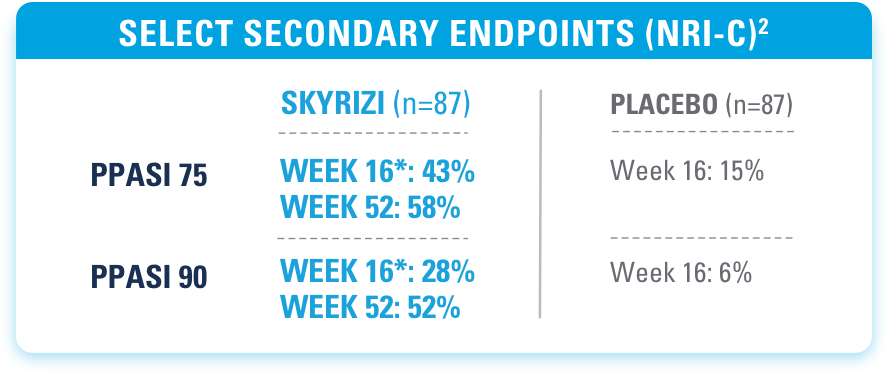
*Ranked secondary endpoints.
52%
(n=86)
Placebo 30% (n=81)
76%
(n=75)
MEAN PPASI SCORES AT BASELINE (SD)2
SKYRIZI (n=87): 22.5 (13.6) PLACEBO (n=87): 22.5 (12.1)
LIMITATIONS:
PPASI 75 and 90 at Week 52 and mean change in PPASI were non-ranked endpoints and not adjusted for multiplicity. Therefore, treatment differences cannot be regarded as statistically significant.
OPEN-LABEL (OL) LIMITATION:
In an open-label study or period, there is potential for enrichment; awareness of active treatment may cause bias to overall treatment effect.
AO DISCLOSURE:
In an as observed analysis (AO) missing visit data were excluded from calculations for that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug, initiation of concomitant medication, or rescue medication. The same patient may not have a response at each timepoint.
STUDY DESIGN:
IMMprint was a Phase 3b, multicenter, randomized, double-blind, parallel-group, placebo-controlled, 52-week study that assessed the safety and efficacy of SKYRIZI (150 mg) vs placebo for the treatment of moderate to severe plaque psoriasis in patients with palmoplantar (non-pustular) involvement. The primary endpoint evaluated the proportion achieving ppIGA of 0 or 1 with ≥2-point reduction from baseline at Week 16. Additional ranked secondary endpoints included PPASI 75, PPASI 90, sPGA 0/1, and PPASI 100 at Week 16.2
PPASI=Palmoplantar Psoriasis Area and Severity Index; ppIGA=Palmoplantar Psoriasis Investigator's Global Assessment; SD=Standard Deviation; sPGA=static Physician's Global Assessment.
SEE ADDITIONAL Ps DATA IN CHALLENGING AREAS
FROM A SUBGROUP ANALYSIS OF UltlMMa-1 AND UltlMMa-2 BELOW
In a subgroup analysis of UltlMMa-1 and UltlMMa-2
94%
IMPROVEMENT
IN SCALP PSORIASIS
(MEAN CHANGE IN PSSI FROM BASELINE)
n=528
66%
IMPROVEMENT IN
NAIL PSORIASIS
(MEAN CHANGE IN NAPSI FROM BASELINE)
n=361
(MEAN CHANGE IN PPASI FROM BASELINE) n=184
MEAN PATIENT SCORES AT BASELINE (SD)
PSSI
18.2 (14.7)
PPASI
2.42 (6.0)
NAPSI
13.6 (18.4)
LIMITATIONS:
Mean changes in PSSI, PPASI, and NAPSI were all pre-specified, non-ranked endpoints and were not adjusted for multiplicity. Therefore, treatment differences cannot be regarded as statistically significant.
Based on analysis of integrated data from UltIMMa-1 and UltIMMa-2 at Week 52 of patients receiving SKYRIZI who had a baseline score of >0 on NAPSI, PSSI, and PPASI, respectively. Missing NAPSI, PSSI, and PPASI data were imputed as last observation carried forward (LOCF).3
STUDY DESIGN:
UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis.1,4
LOCF=Last Observation Carried Forward; PSSI=Psoriasis Scalp Severity Index; PPASI=Palmoplantar Psoriasis Area and Severity Index; NAPSI=Nail Psoriasis Severity Index; SD=Standard Deviation.
4 doses per year
3-month dosing after 2 initiation doses at
Weeks 0 and 4 (150 mg/dose)1
Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults.
Hypersensitivity Reactions
SKYRIZI® (risankizumab-rzaa) is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab-rzaa or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of SKYRIZI. If a serious hypersensitivity reaction occurs, discontinue SKYRIZI and initiate appropriate therapy immediately.
Infection
SKYRIZI may increase the risk of infection. Do not initiate treatment with SKYRIZI in patients with a clinically important active infection until it resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing SKYRIZI. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, closely monitor and discontinue SKYRIZI until the infection resolves.
Tuberculosis (TB)
Prior to initiating treatment with SKYRIZI, evaluate for TB infection and consider treatment in patients with latent or active TB for whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SKYRIZI treatment. Do not administer SKYRIZI to patients with active TB.
Administration of Vaccines
Avoid use of live vaccines in patients treated with SKYRIZI. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating SKYRIZI, complete all age appropriate vaccinations according to current immunization guidelines.
Adverse Reactions
Most common (≥1%) adverse reactions associated with SKYRIZI include upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
In psoriatic arthritis phase 3 trials, the incidence of hepatic events was higher with SKYRIZI compared to placebo.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
Please see Full Prescribing Information.
US-SKZD-210594
REFERENCES
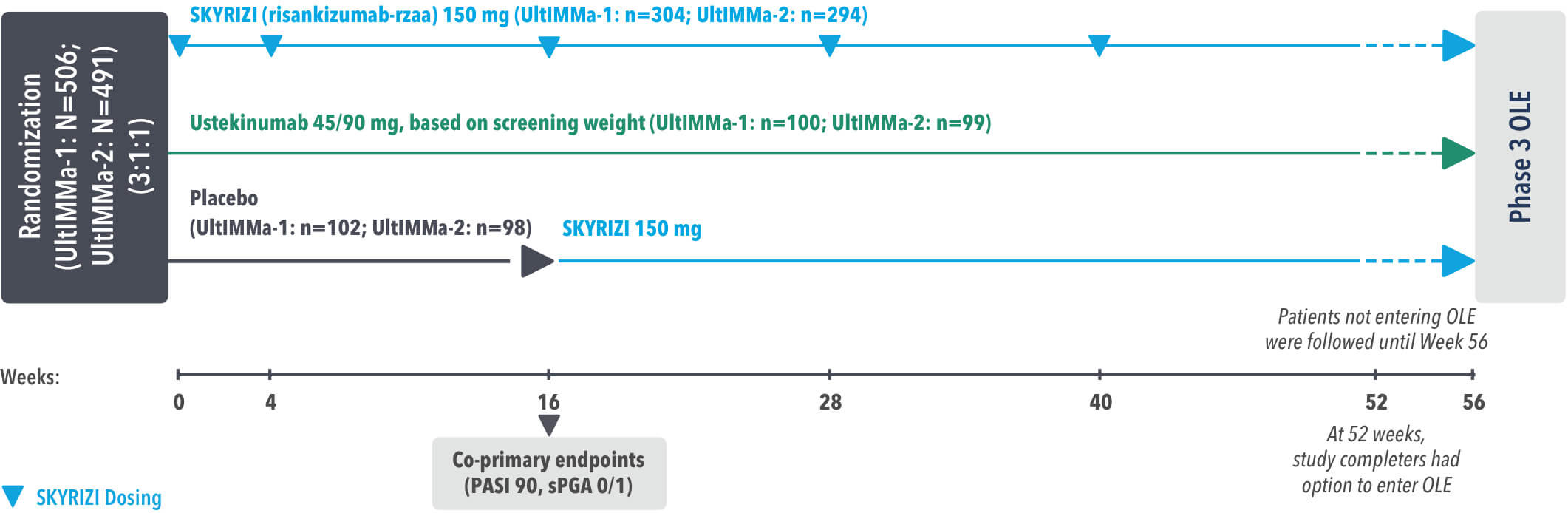

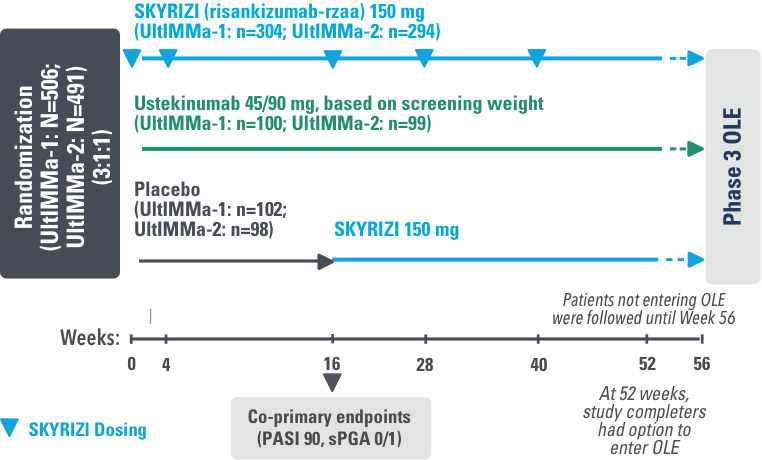
UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References
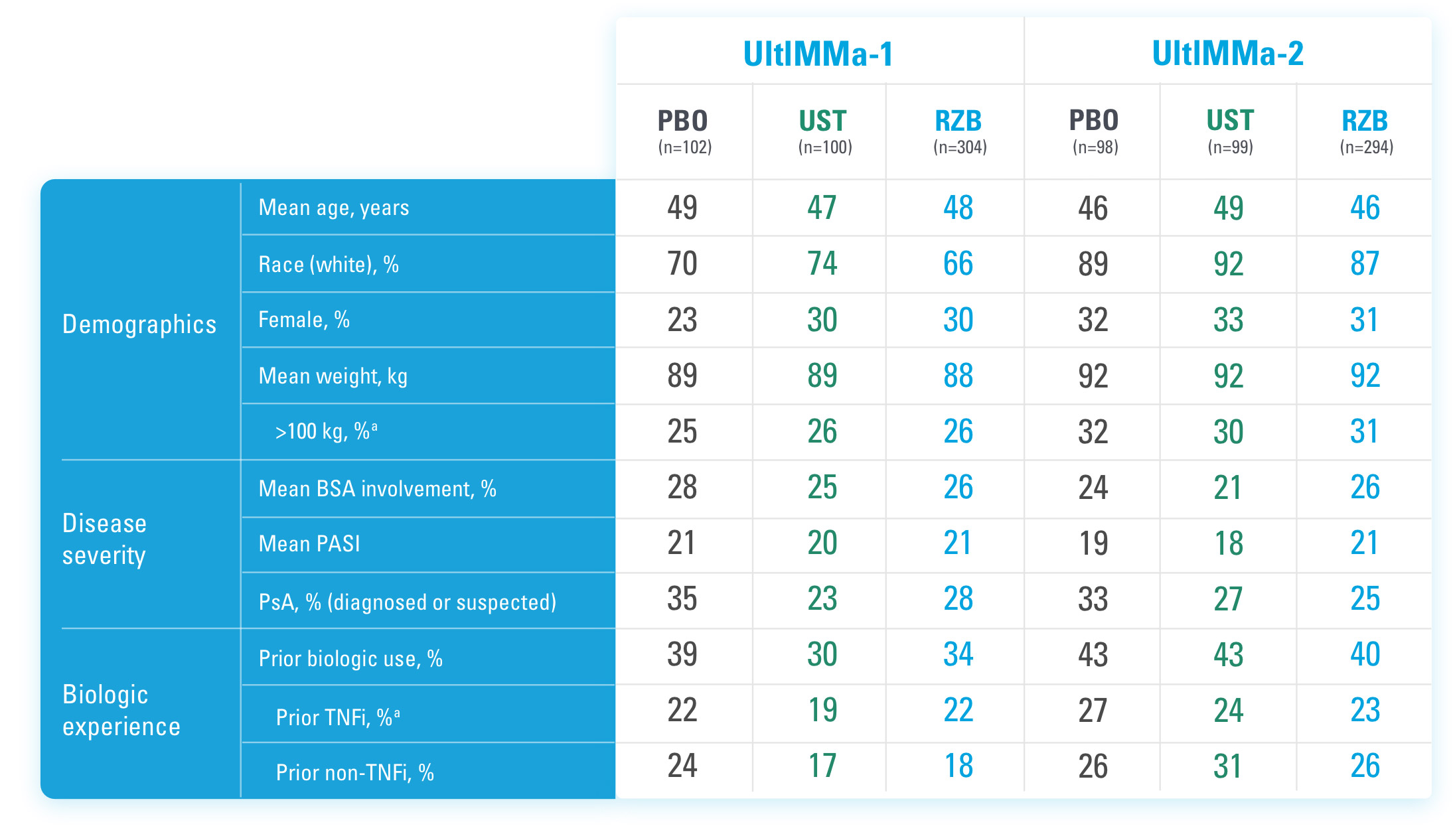

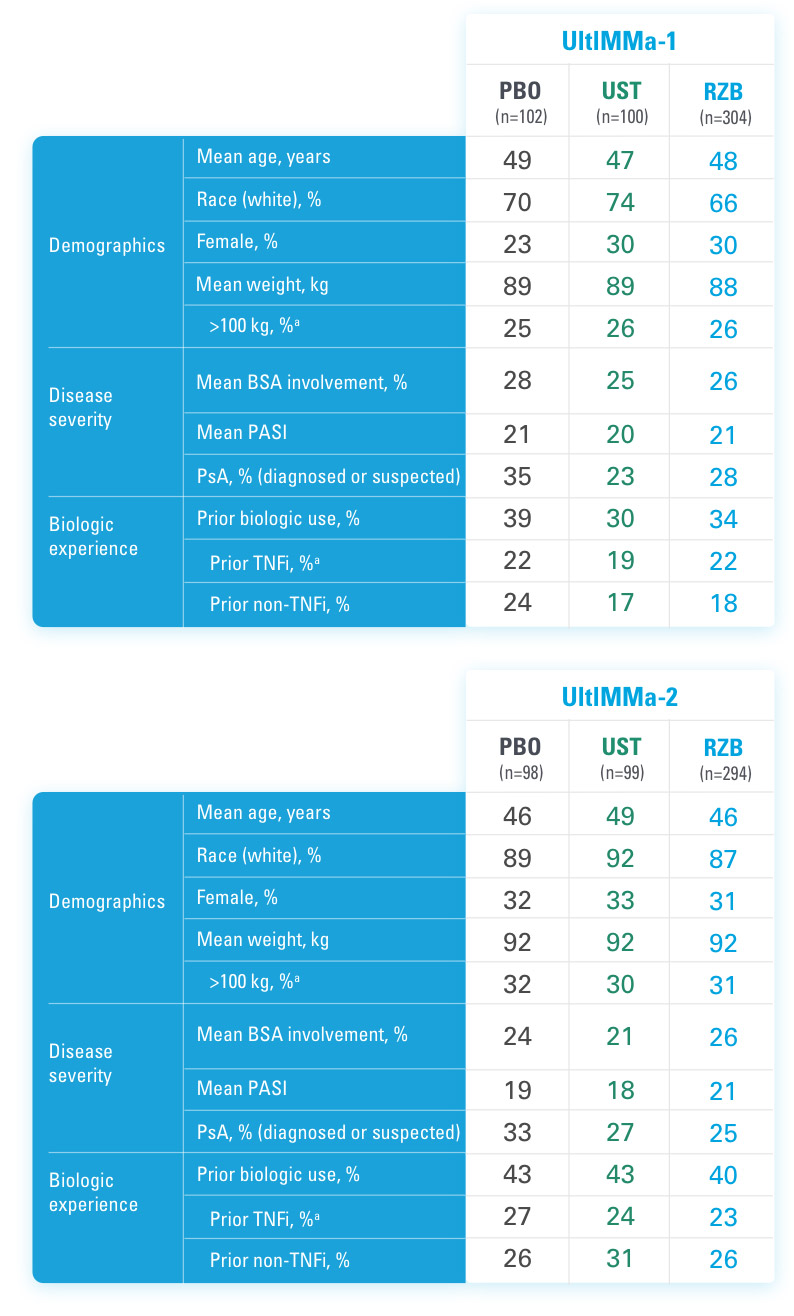
BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459



UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459



IMMprint was a Phase 3b, multicenter, randomized, double-blind, parallel-group, placebo-controlled, 52-week study that assessed the safety and efficacy of SKYRIZI vs placebo for the treatment of moderate to severe plaque psoriasis in patients with palmoplantar (non-pustular) involvement. SKYRIZI (150 mg) was given as a subcutaneous injection at Weeks 0, 4, and every 12 weeks thereafter. At Week 16, patients on placebo were switched to SKYRIZI Q12W.
At least 50% of the overall study population was comprised of subjects with baseline BSA ≥10%.
Primary endpoint
BSA=body surface area
PPASI=Palmoplantar Psoriasis Area and Severity Index
ppIGA=Palmoplantar Investigator's Global Assessment
Ps=plaque psoriasis
PsA=psoriatic arthritis
sPGA=static Physician's Global Assessment
Ranked secondary endpoints
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References
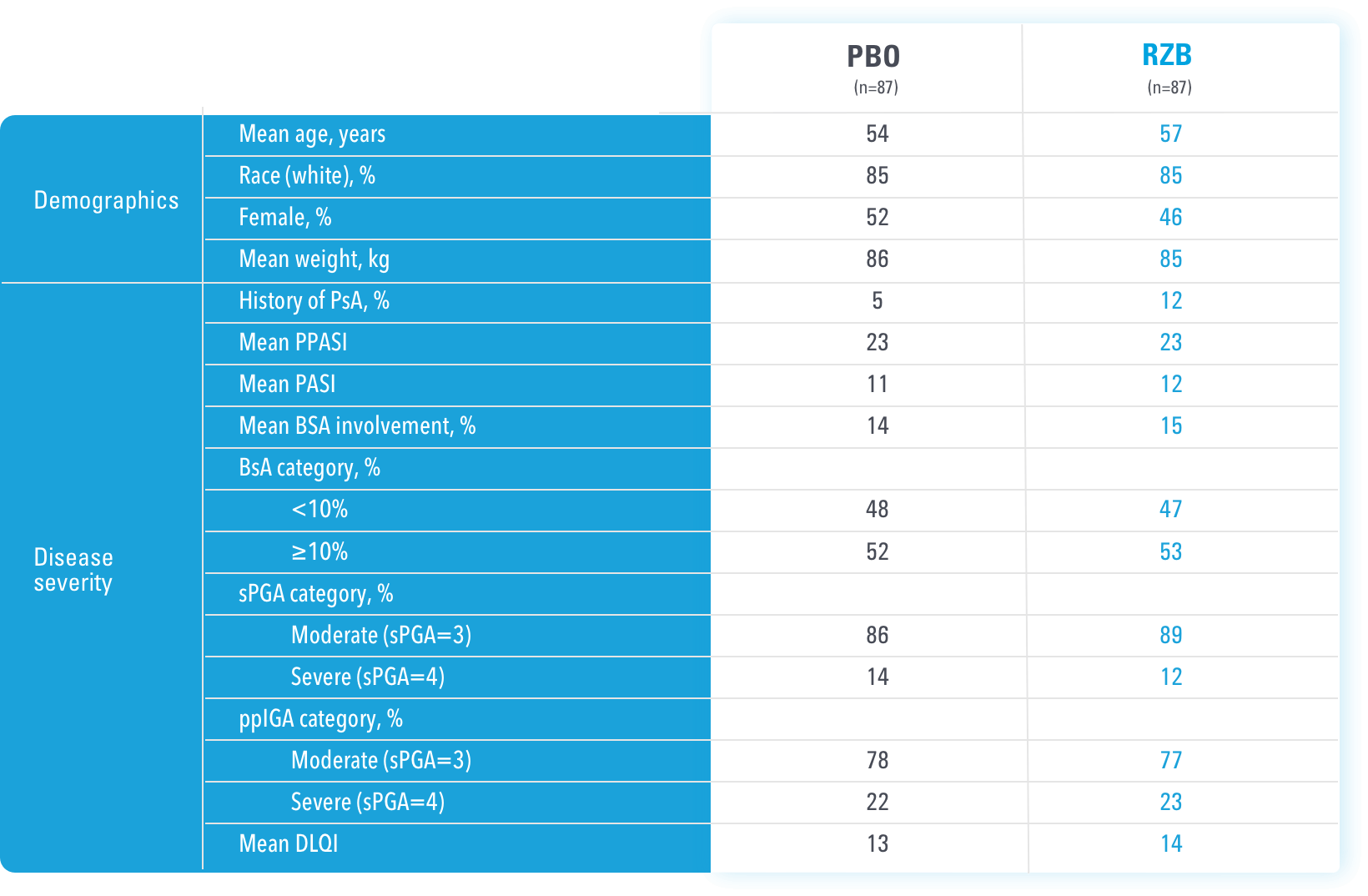

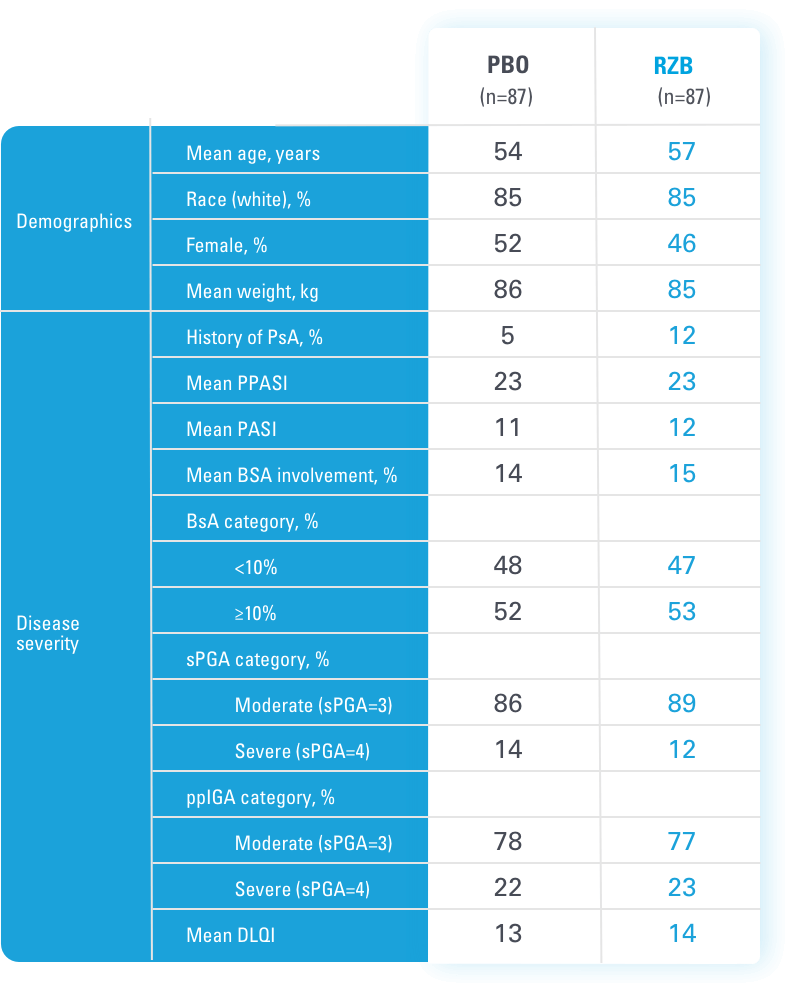
BSA=body surface area
DLQI=Dermatology Life Quality Index
PASI=Psoriasis Area and Severity Index
PBO=placebo
PPASI=Palmoplantar Psoriasis Area and Severity Index
ppIGA=Palmoplantar Psoriasis Investigator's Global Assessment
Ps=plaque psoriasis
PsA=psoriatic arthritis
RZB=risankizumab-rzaa
sPGA=static Physician's Global Assessment
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459
A STUDY EVALUATING PALMOPLANTAR INVOLVEMENT IN PATIENTS WITH MODERATE TO SEVERE Ps

AE=adverse event
IBD=inflammatory bowel disease
MACE=major adverse cardiac events
NMSC=non-melanoma skin cancer
Initially evaluate for tuberculosis (TB) and instruct patients to report signs and symptoms of infection.
no routine lab monitoring required during treatment.1
no labeled liver testing required for initiation or during treatment for Ps or PsA.1
Warnings and precautions include hypersensitivity, infections, tuberculosis, and immunizations.
NO LABELED WARNINGS OR PRECAUTIONS ON MALIGNANCY, IBD, DEPRESSION, OR CANDIDIASIS.1
OPEN-LABEL LIMITATION:
In an open-label study or period, there is potential for enrichment; awareness of active treatment may cause bias to overall treatment effect.
References



IMMprint was a Phase 3b, multicenter, randomized, double-blind, parallel-group, placebo-controlled, 52-week study that assessed the safety and efficacy of SKYRIZI vs placebo for the treatment of moderate to severe plaque psoriasis in patients with palmoplantar (non-pustular) involvement. SKYRIZI (150 mg) was given as a subcutaneous injection at Weeks 0, 4, and every 12 weeks thereafter. At Week 16, patients on placebo were switched to SKYRIZI Q12W.
At least 50% of the overall study population was comprised of subjects with baseline BSA ≥10%.
Primary endpoint
BSA=body surface area
PPASI=Palmoplantar Psoriasis Area and Severity Index
ppIGA=Palmoplantar Investigator's Global Assessment
Ps=plaque psoriasis
PsA=psoriatic arthritis
sPGA=static Physician's Global Assessment
Ranked secondary endpoints
Key inclusion criteria
References



BSA=body surface area
DLQI=Dermatology Life Quality Index
PASI=Psoriasis Area and Severity Index
PBO=placebo
PPASI=Palmoplantar Psoriasis Area and Severity Index
ppIGA=Palmoplantar Psoriasis Investigator's Global Assessment
Ps=plaque psoriasis
PsA=psoriatic arthritis
RZB=risankizumab-rzaa
sPGA=static Physician's Global Assessment
References
US-SKZD-230756
In patients who achieved PASI 90 at Week 16...
88%
maintained PASI 90
at Week 521,2
(n=398/450)
MAINTENANCE OF RESPONSE
INTEGRATED ANALYSIS OF UltIMMa-1 AND UltIMMa-2
References
US-SKZD-230756
In patients who achieved PASI 100 at Week 16...
80%
maintained PASI
100 at Week 521
(n=206/258)
MAINTENANCE OF RESPONSE
INTEGRATED ANALYSIS OF UltIMMa-1 AND UltIMMa-2
Reference
US-SKZD-230756
MAINTENANCE OF RESPONSE,
ALL DATA ARE AS OBSERVED1
OLE limitations: In an open-label extension, there is a potential for enrichment of the long-term data in the remaining
patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
AO DISCLOSURE: In an as observed analysis (AO) missing visit data were excluded from calculations for that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug, initiation of concomitant medication, or rescue medication. The same patient may not have a response at each timepoint.
Reference
US-SKZD-230756



UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459
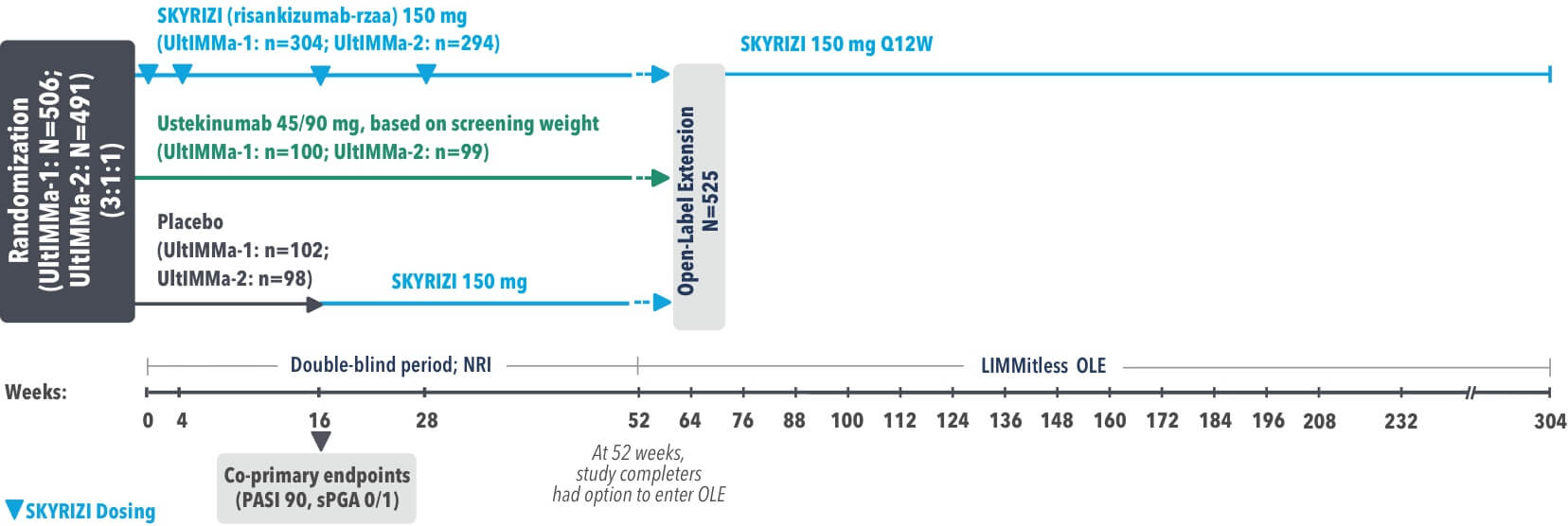



UltIMMa-1 and UltIMMa-2 were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI compared to placebo or ustekinumab in adult patients with moderate to severe plaque psoriasis over 52 weeks. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
LIMMitless is an ongoing, single-arm, multicenter, open-label extension of Phase 2 and 3 studies evaluating the long-term efficacy and safety of SKYRIZI (150 mg). Patients who completed UltIMMa-1 or -2, IMMvent, or IMMhance were eligible to participate.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
NRI=Nonresponder Imputation
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-230756
US-SKZD-220459
US-SKZD-220459



UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459




UltIMMa-1 and UltIMMa-2 were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI compared to placebo or ustekinumab in adult patients with moderate to severe plaque psoriasis over 52 weeks. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
LIMMitless is an ongoing, single-arm, multicenter, open-label extension of Phase 2 and 3 studies evaluating the long-term efficacy and safety of SKYRIZI (150 mg). Patients who completed UltIMMa-1 or -2, IMMvent, or IMMhance were eligible to participate.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
NRI=Nonresponder Imputation
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-230756
On January 21, 2022, the Prescribing Information and Medication Guide for SKYRIZI (risankizumab-rzaa) was updated to add a Contraindication and a new Warning and Precaution for Serious Hypersensitivity Reactions.
The relevant sections of the Prescribing Information read as follows:
4 CONTRAINDICATIONS
SKYRIZI is contraindicated in patients with a history of serious hypersensitivity reaction to
risankizumab-rzaa or any of the excipients.
5 WARNINGS AND PRECAUTIONS
Section 5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported with use of SKYRIZI. If a serious hypersensitivity reaction occurs, discontinue SKYRIZI and initiate appropriate therapy immediately.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients to discontinue SKYRIZI and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
The following information on important labeling revisions does not include all changes; please refer to the SKYRIZI Full Prescribing Information.
INDICATIONS AND IMPORTANT SAFETY INFORMATION FOR SKYRIZI® (risankizumab-rzaa)1 Indications Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults. Crohn's Disease: SKYRIZI is indicated for the treatment of moderately to severely active Crohn's disease in adults. Ulcerative Colitis: SKYRIZI is indicated for the treatment of moderately to severely active ulcerative colitis in adults. |
Important Safety Information
Hypersensitivity Reactions
SKYRIZI® (risankizumab-rzaa) is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab-rzaa or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of SKYRIZI. If a serious hypersensitivity reaction occurs, discontinue SKYRIZI and initiate appropriate therapy immediately.
Infection
SKYRIZI may increase the risk of infection. Do not initiate treatment with SKYRIZI in patients with a clinically important active infection until it resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing SKYRIZI. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, closely monitor and discontinue SKYRIZI until the infection resolves.
Tuberculosis (TB)
Prior to initiating treatment with SKYRIZI, evaluate for TB infection and consider treatment in patients with latent or active TB for whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SKYRIZI treatment. Do not administer SKYRIZI to patients with active TB.
Hepatotoxicity in Treatment of Inflammatory Bowel Disease
Drug-induced liver injury was reported in a patient with Crohn’s disease who was hospitalized for a rash during induction dosing of SKYRIZI. For the treatment of Crohn's disease and ulcerative colitis, evaluate liver enzymes and bilirubin at baseline and during induction (12 weeks); monitor thereafter according to routine patient management. Consider an alternate treatment for patients with evidence of liver cirrhosis. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Instruct your patient to seek immediate medical attention if they experience symptoms suggestive of hepatic dysfunction.
Administration of Vaccines
Avoid use of live vaccines in patients treated with SKYRIZI. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating SKYRIZI, complete all age-appropriate vaccinations according to current immunization guidelines.
Adverse Reactions
Most common (≥1%) adverse reactions associated with SKYRIZI in plaque psoriasis and psoriatic arthritis include upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
In psoriatic arthritis phase 3 trials, the incidence of hepatic events was higher with SKYRIZI compared to placebo.
Most common (>3%) adverse reactions associated with SKYRIZI in Crohn’s disease are upper respiratory infections, headache, and arthralgia in induction and arthralgia, abdominal pain, injection site reactions, anemia, pyrexia, back pain, arthropathy, and urinary tract infection in maintenance.
Most common (≥3%) adverse reactions associated with SKYRIZI in ulcerative colitis are arthralgia in induction, and arthralgia, pyrexia, injection site reactions, and rash in maintenance.
Lipid Elevations: Increases from baseline and increases relative to placebo were observed at Week 4 and remained stable to Week 12 in patients treated with SKYRIZI in Crohn’s disease. Lipid elevations observed in patients with ulcerative colitis were similar to those in Crohn's disease.
Dosage Forms and Strengths: SKYRIZI (risankizumab-rzaa) is available in a 150 mg/mL prefilled syringe and pen, a 600 mg/10 mL single-dose vial for intravenous infusion, and a 180 mg/1.2 mL or 360 mg/2.4 mL single-dose prefilled cartridge with on-body injector.
INDICATIONS
Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults.
Crohn's Disease: SKYRIZI is indicated for the treatment of moderately to severely active Crohn's disease in adults.
Ulcerative Colitis: SKYRIZI is indicated for the treatment of moderately to severely active ulcerative colitis in adults.
Please see Full Prescribing Information.
US-SKZG-240258
US-SKZD-210908
SKYRIZI (risankizumab-rzaa)
Injection Training Video
Hi, it’s good to be here with you. I’m Kate. And you are 1 of the many people who have been prescribed SKYRIZI. After starting on SKYRIZI, there were 2 directions you could go: continue with in-office injections or inject at home.
You had a discussion with your doctor, and have decided to inject at home.
I’m going to show you how to do that...step by step, and address any questions you may have.
Still, there are probably a million things you’d rather be doing right now than learning about injecting your medicine. I get it. But this video will be helpful. So, stay with it. Because, well, there’s more to making SKYRIZI a part of your life than just learning how to inject.
When you sign up for Skyrizi Complete, you get a dedicated Nurse Ambassador.
They will get to know you and help you start and stay on track with your treatment plan.
So, let’s get started.
Leave SKYRIZI at room temperature for 15 to 30 minutes before injecting.
If you’re a little nervous, I totally get it. It may help you relax if you watch or listen to something that is soothing.
First, wash your hands. Then get the things you need, and settle into a place where you feel relaxed.
Now I’ll take you through the process step by step.
Make sure you have everything you need laid out:
2 alcohol swabs that are included in the package,
2 cotton balls, you can also use gauze pads,
your sharps container, which ensures your syringes are disposed of safely,
and of course, your 2 SKYRIZI prefilled syringes.
For 1 full dose, 2 injections are required.
We’re going to break the injection process down into 4 simple steps.
Let’s call them the 4 Ps.
Pick the injection site.
Prepare the syringe.
Pinch the skin.
And Push the plunger in.
There’s an “R” too for “Repeat.” Remember, 1 dose is 2 injections.
So, you will need to repeat the same injection steps for the second injection.
Pick the injection site—your left or right thigh or your stomach.
When you are using your second syringe, pick an injection site at least 1 inch away from the first site. Do not inject into the same site.
If you choose your lower stomach area, make sure you inject at least 2 inches away from your belly button.
Wipe the injection site in a circular motion with the alcohol swab (before both injections). Don’t inject through clothes, or into skin that doesn’t look normal.
Start with 1 syringe for the first injection. Now prepare the syringe.
Holding the syringe with the needle pointing down, check the liquid in the syringe.
It is normal to see 1 or more bubbles in the window.
The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
DO NOT use if the liquid is cloudy or contains flakes or large particles.
To remove the needle cover, hold the syringe in 1 hand.
With the other hand, gently pull the needle cover straight off and throw it away.
You may see a drop of liquid at the end of the needle. This is normal.
DO NOT touch the needle with your fingers or let the needle touch anything.
For this demonstration, I’ll be using a practice pad.
Hold the body of the prefilled syringe in 1 hand between the thumb and index finger.
Gently pinch the area of cleaned skin with your other hand and hold it firmly.
Insert the needle into the skin at about a 45-degree angle using a quick, short movement. Hold the angle steady.
Slowly push the plunger all the way in until all of the liquid is injected and the syringe is empty.
Pull the needle out of the skin while keeping the syringe at the same angle. Release the plunger and allow the syringe to move up until the entire needle is covered by the needle guard.
The syringe needle guard will not activate unless all the liquid has been injected.
Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
DO NOT rub the injection site. You may have slight bleeding. This is normal.
Now that the syringe is empty, drop it into the sharps container.
Repeat these injection steps for the second syringe immediately following your first injection. Do not inject into the same site.
Be sure to pick a new site at least 1 inch away from your first injection.
In a nutshell here’s what you did.
You picked the injection site, prepared the syringe, pinched the skin, and pushed the plunger in. Then you did it again, using the second syringe.
Okay, so now you know how to inject.
After your starter doses at Week 0 and Week 4, SKYRIZI is dosed quarterly. That’s just 4 times a year or 1 dose for each season.
It’s important you don’t forget.
So, make sure you place reminders for ordering your medication and note your injection day on your calendar. You can also do this through the Skyrizi Complete App.
The Skyrizi Complete Sharps Disposal and Mail-back Service ensures your syringes are disposed of safely and in a socially responsible way.
There’s a bit to remember.
But you’ll get the hang of it. And you can always refer to this video or call us.
You’ve got this.
US-SKZD-210080
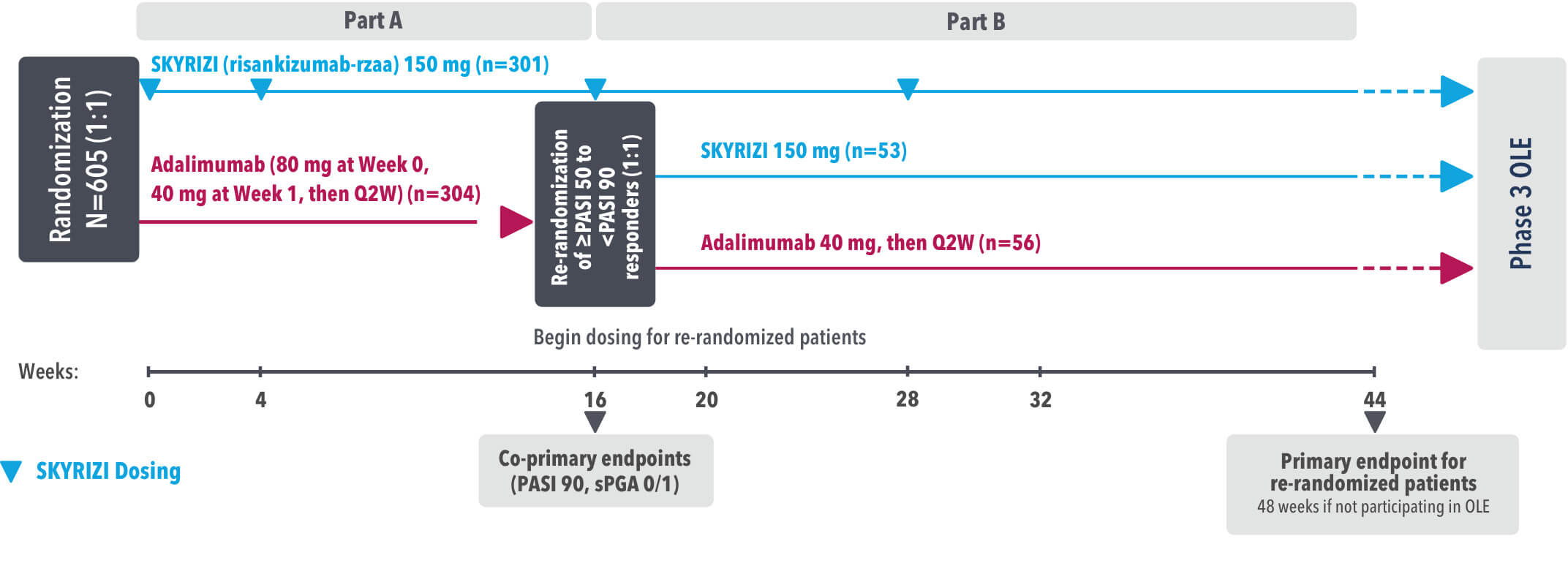

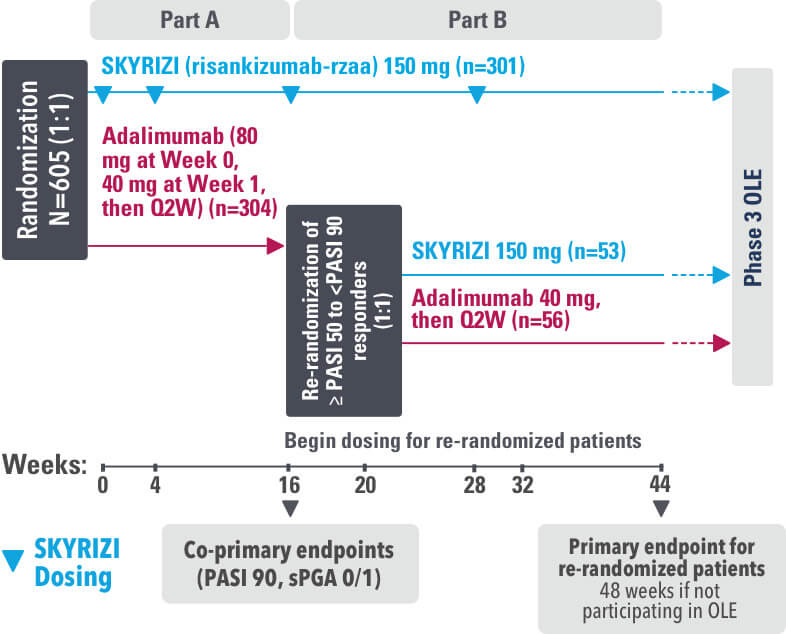
IMMvent was a Phase 3, randomized, double-blind, double-dummy, active-controlled study to evaluate the efficacy and safety of SKYRIZI (150 mg) compared to HUMIRA® (adalimumab) in adult patients with moderate to severe plaque psoriasis over 44 weeks.
Patients treated with adalimumab during Part A:
Part A
In the first phase, patients were randomized 1:1 to either SKYRIZI (150 mg), given as a subcutaneous injection at Weeks 0 and 4 and every 12 weeks thereafter or HUMIRA, given as a subcutaneous injection, with an initial dose of 80 mg followed by 40 mg every other week starting 1 week after the initial dose over 44 weeks.
The Part A co-primary endpoints were
Part B
Patients originally randomized to SKYRIZI received it throughout the study (Parts A & B). Among patients originally randomized to receive HUMIRA, those with a PASI 50 but less than PASI 90 response were re-randomized 1:1 to switch to SKYRIZI or continue HUMIRA.
The Part B primary endpoint was
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
Q2W=Once every 2 weeks
sPGA=static Physician's Global Assessment
Click to see HUMIRA® (adalimumab) Indication and Important Safety Information, including BOXED WARNING for Serious Infections and Malignancy
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
Q2W=Once every 2 weeks
sPGA=static Physician's Global Assessment
Reference
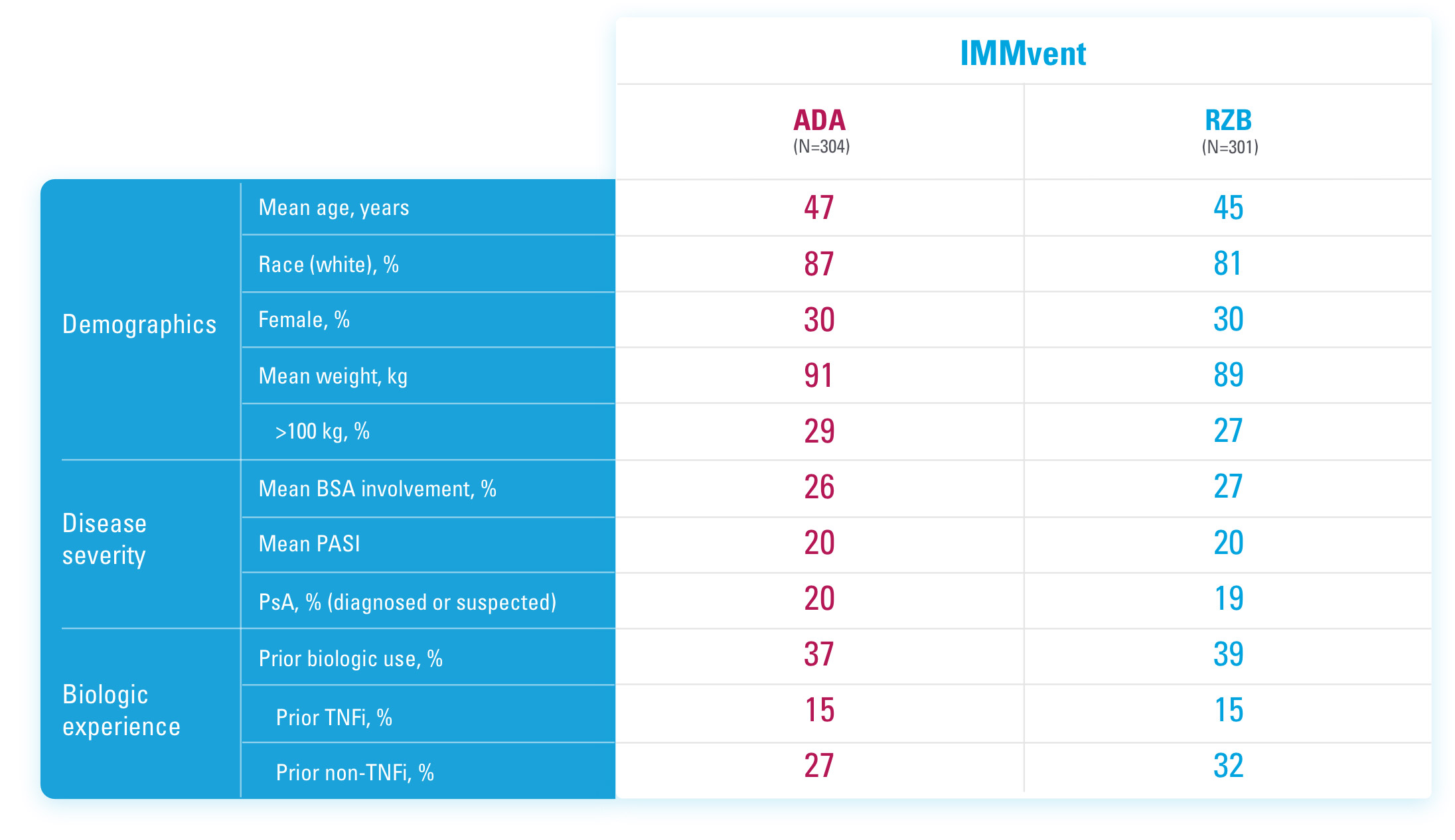

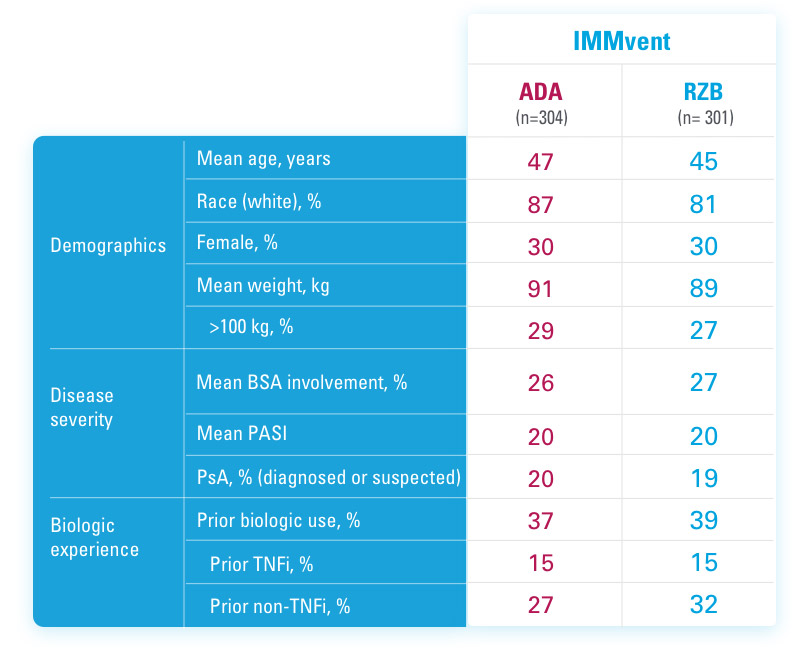
ADA=adalimumab
BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
References
US-SKZD-220459
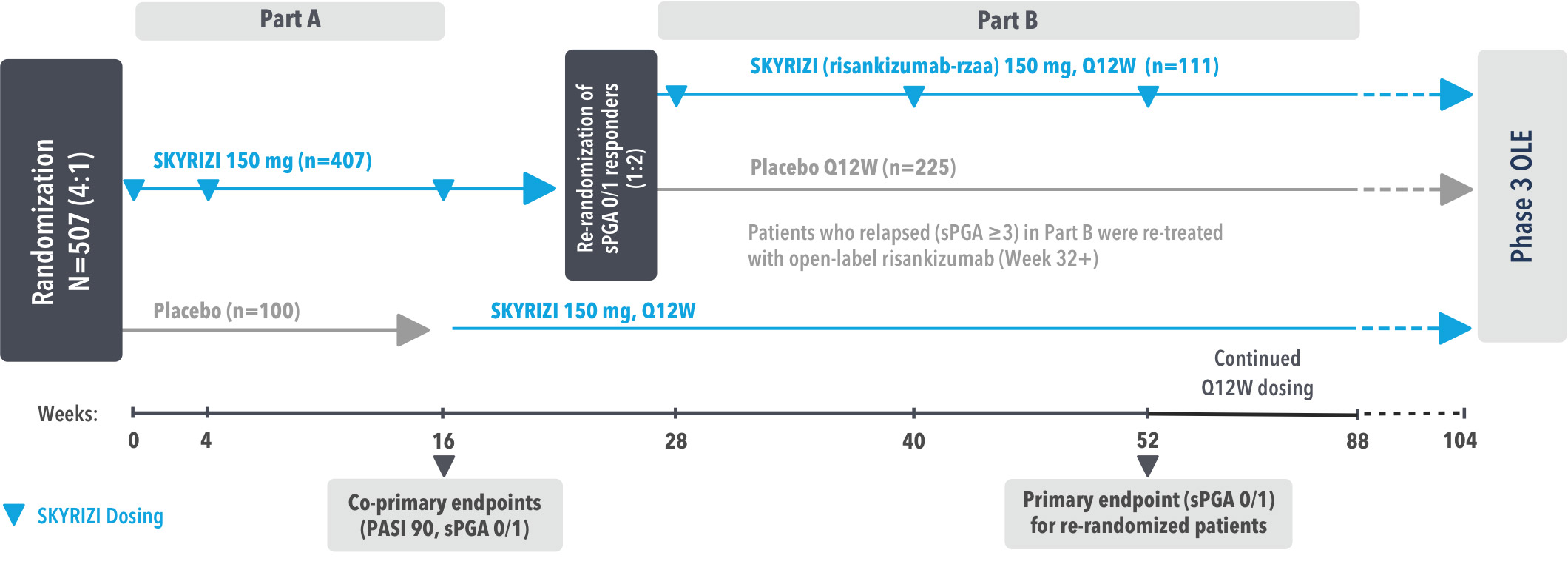

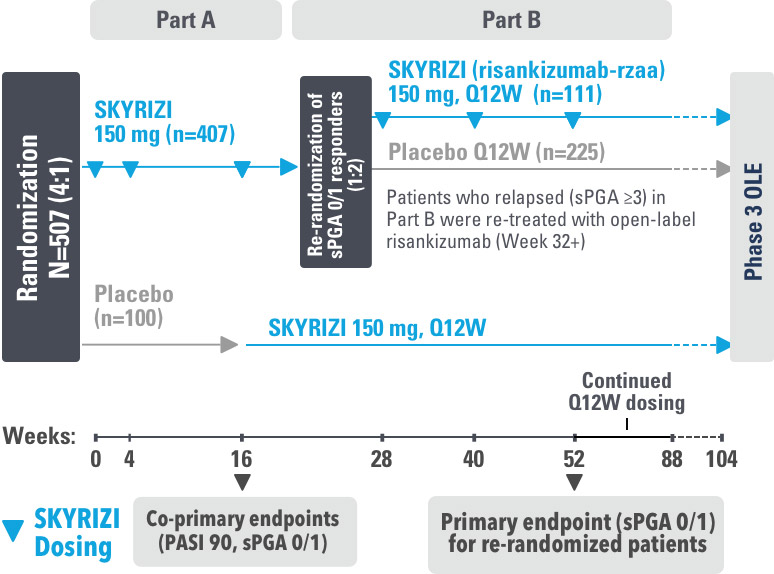
IMMhance was a Phase 3, multicenter, randomized, double-blind, placebo-controlled study to evaluate the impact of treatment withdrawal and re-treatment of SKYRIZI compared to placebo in adult patients with moderate to severe plaque psoriasis.
Part A
In the first phase, patients were randomized 4:1 to SKYRIZI (150 mg), given as a subcutaneous injection at baseline, 4 weeks later, and every 12 weeks thereafter, or placebo.
The Part A co-primary endpoints were
Part B
In the second phase of this study (Week 28 through Week 104), patients originally randomized to SKYRIZI who achieved sPGA 0/1 at Week 28 were re-randomized (1:2) to SKYRIZI (maintenance) or placebo (withdrawal). Beginning at Week 28, patients with sPGA ≥2 continued on SKYRIZI (150 mg) once every 12 weeks up to Week 88, with a final follow-up at Week 104.
The Part B primary endpoint was
Key inclusion criteria
Key secondary endpoints included
DLQI=Dermatology Life Quality Index
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 75=≥75% improvement in Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
Q12W=Once every 12 weeks
sPGA=static Physician's Global Assessment
References
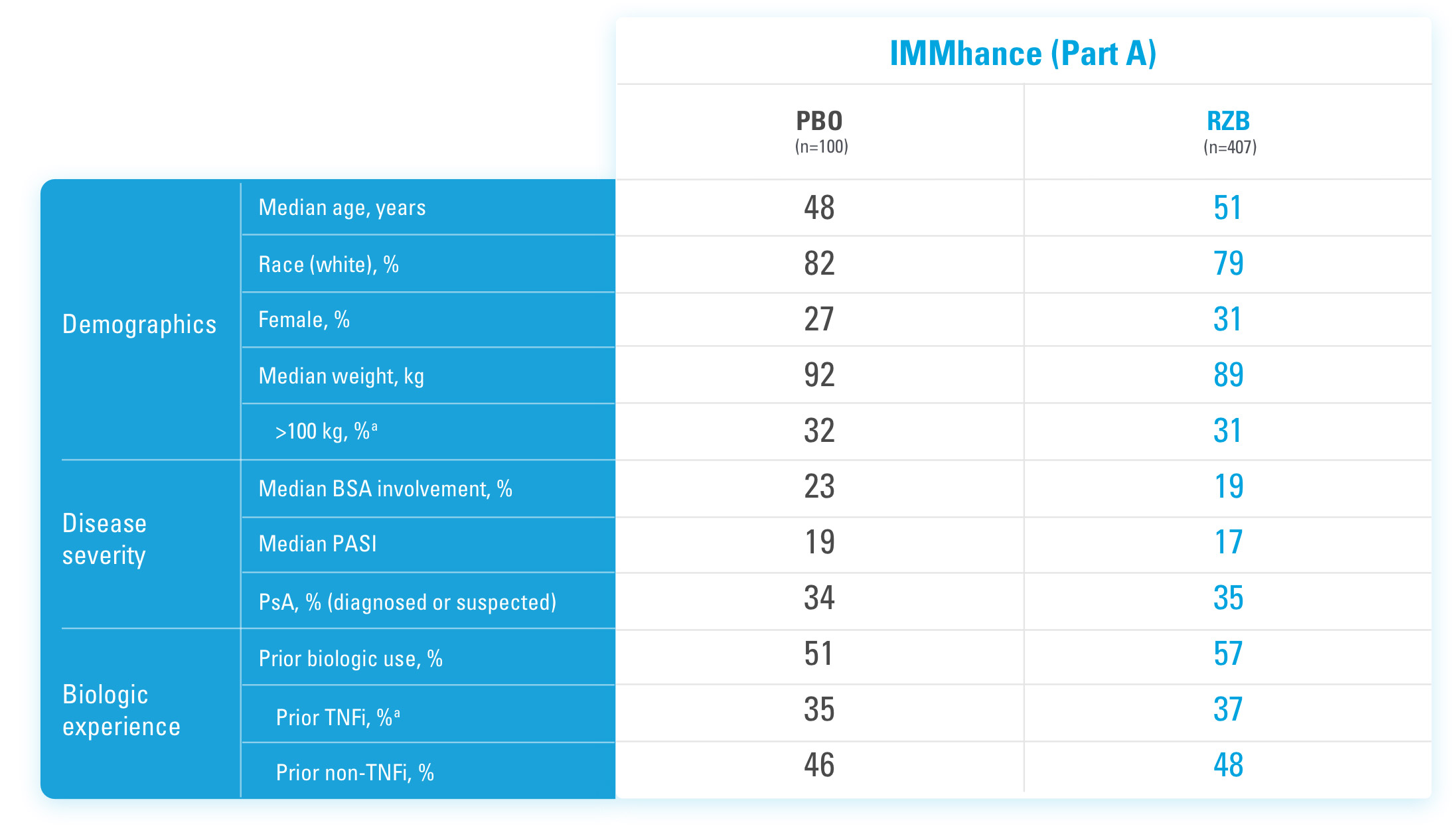

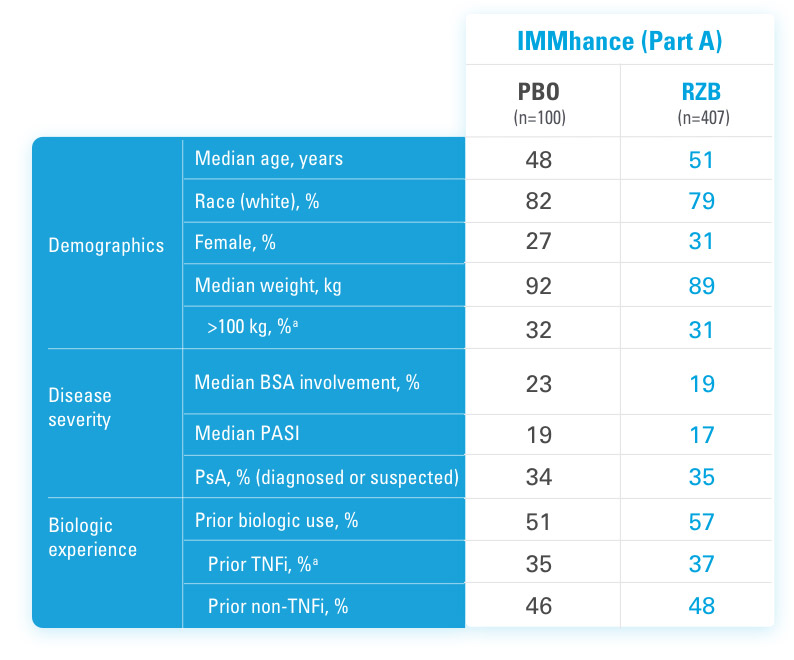
BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
aStratification factors at randomization.
References
US-SKZD-220459
YOU ARE LEAVING SKYRIZIHCP.COM
You are leaving the SKYRIZIHCP.com website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of SKYRIZIHCP.com or AbbVie.
US-SKZD-220457
YOU ARE LEAVING SKYRIZIHCP.COM
You are about to enter a site that is for U.S. Healthcare Professionals Only.
By selecting "Continue" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
US-SKZ-220057
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
YOU ARE LEAVING SKYRIZIHCP.COM
You are leaving the SKYRIZIHCP.com website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of SKYRIZIHCP.com or AbbVie.
US-SKZD-220459
YOU ARE LEAVING SKYRIZIHCP.COM
You are leaving the SKYRIZIHCP.com website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of SKYRIZIHCP.com or AbbVie.
US-SKZD-220459
Bridge Program Update:
If your eligible, commercially insured patient receives an insurance denial due to step therapy requirements and you wish to appeal based on medical necessity*:
*Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for SKYRIZI® (risankizumab-rzaa) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for SKYRIZI at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.
US-SKZD-230756



UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. SKYRIZI (150 mg) was given as 2 subcutaneous injections at Weeks 0, 4, 16, 28, and 40. Patients were randomized 3:1:1 to receive SKYRIZI, ustekinumab, or placebo. At Week 16, patients on placebo were switched to SKYRIZI.
Active comparator
The active comparator (ustekinumab) used for these studies was sourced from the European Union.
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
The co-primary endpoints were
Key secondary endpoints included
Key inclusion criteria
OLE=Open-Label Extension
PASI=Psoriasis Area and Severity Index
PASI 90=≥90% improvement in Psoriasis Area and Severity Index
PASI 100=100% improvement in Psoriasis Area and Severity Index
sPGA=static Physician's Global Assessment
References



BSA=Body Surface Area
PASI=Psoriasis Area and Severity Index
PBO=Placebo
PsA=Psoriatic Arthritis
RZB=risankizumab-rzaa
TNFi=Tumor Necrosis Factor Inhibitor
UST=ustekinumab
BSA=Body Surface Area; PASI=Psoriasis Area and Severity Index; PBO=Placebo; PsA=Psoriatic Arthritis; RZB=risankizumab-rzaa; TNFi=Tumor Necrosis Factor Inhibitor; UST=ustekinumab
aStratification factors at randomization.
Reference
US-SKZD-220459
High Levels of Durable Skin Clearance
Uncovering Clearance
Watch Jeff Crowley, MD, and Jennifer Soung, MD, walk through the PASI 90 and PASI 100 data through 1 year of treatment with SKYRIZI.
Please see Safety Considerations at the end of this video and review the full Prescribing Information available at SKYRIZIHCP.com.
Dr. Soung: Biologics have helped many patients with moderate to severe plaque psoriasis by offering additional treatment options for clearing skin.
Dr. Crowley: Today, Dr. Soung and I are going to share with you the key features of SKYRIZI and how it's helped patients like ours.
In 2 pivotal trials, UltIMMa-1 and UltIMMa-2, SKYRIZI was proven to deliver high levels of clearance in patients with moderate to severe plaque psoriasis, with 4 doses a year after 2 initial doses.
The co-primary endpoints in the trials were PASI 90 and sPGA O or 1 at Week 16 for SKYRIZI versus placebo. SKYRIZI met both co-primary endpoints in each trial. In addition to placebo, SKYRIZI was evaluated against a biologic active comparator, ustekinumab, which was sourced from the European Union. Comparability between non–US-approved ustekinumab and US-approved ustekinumab has not been established.
Dr. Soung: Let's take a look at SKYRIZl's 52-week data.
Here we see in this chart SKYRIZl's PASI 90 efficacy over 52 weeks from the pooled UltlMMa-1 and -2 trials. I want to bring your attention to the dosing schedule you see on the x-axis, as indicated by an arrow.
After 2 doses of SKYRIZI, 75% of patients achieved PASI 90 at Week 16, compared to 4% for placebo. The dosing schedule is every 12 weeks for maintenance. At 1 year after 5 doses, the proportion of patients achieving PASI 90 increases to 81%.
In addition, SKYRIZI also maintained response over time. 88% of patients who saw PASI 90 results at Week 16 maintained PASI 90 at Week 52.
Many of my patients tell me that completely clear skin matters. Let's take a look at the PASI 100 responses from the pooled UltlMMa-1 and -2 trials.
43% of SKYRIZl-treated patients achieved PASI 100 at Week 16 after just 2 doses, compared to 1% with placebo. In 1 year, after just 5 doses, 58% of SKYRIZl-treated patients achieved PASI 100.
Dr. Crowley: Safety is another important consideration for patients and providers alike. SKYRIZI has a well-studied safety profile across 4 pivotal trials, with a total of 1,306 patients receiving SKYRIZI. Here we see the rates of adverse events through Week 16. In the trials, the SKYRIZI safety profile was similar to that of EU-sourced ustekinumab.
Rates of adverse events with SKYRIZI through 52 weeks were similar to the safety profile observed during the first 16 weeks.
With SKYRIZI, warnings and precautions include risk of infections, tuberculosis, and avoiding the use of live vaccines. There are no labeled warnings or precautions on malignancy, inflammatory bowel disease, or depression.
In summary, SKYRIZI offers patients with moderate to severe plaque psoriasis the opportunity for high levels of durable skin clearance with a well-studied safety profile, making it an excellent treatment option.
Dr. Soung: The fact that the majority of patients during the trials were completely clear at 1 year is exciting for our patients.
Indication
SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Safety Considerations
SKYRIZI may increase the risk of infection. Instruct patients to report signs or symptoms of clinically important infection during treatment. Should such an infection occur, discontinue SKYRIZI until infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with SKYRIZI. Avoid use of live vaccines in SKYRIZI patients.
Please see additional Important Safety Information at the bottom of this page. Please see full Prescribing Information by clicking the link at the top of this page.
US-SKZD-190187
US-SKZD-200190
Expectation Setting and Mean PASI Data
Clear Conversations
Hear from Cynthia Trickett, PA-C, MPAS, and Jason M. Cheyney, PA-C, MPAS, as they discuss setting patient expectations on efficacy and safety with SKYRIZI.
Please see Safety Considerations at the end of this video and review the full Prescribing Information available at SKYRIZIHCP.com.
Cynthia: When my patients start a biologic, having clear conversations on efficacy and safety are essential. Two common questions I get from patients are "How fast will this treatment work?" and "What are the risks?"
In 2 pivotal trials, UltlMMa-1 and UltlMMa-2, SKYRIZI demonstrated high levels of skin clearance in patients with moderate to severe plaque psoriasis.
The co-primary endpoints in the trials were PASI 90 and sPGA O or 1 at Week 16 for SKYRIZI versus placebo. In both trials, the co-primary endpoints were met.
SKYRIZI patients also saw rapid responses, with results seen as early as Week 4.
Jason: It can be difficult at times for patients to understand PASI 90 data. Another way to help explain clinical trial results to patients is mean PASI.
Mean PASI improvement is the average improvement in skin clearance from baseline—it's what the average patient experienced in the clinical trials, measured at set time periods.
When discussing efficacy with my patients starting SKYRIZI, I incorporate mean PASI, which was a pre-specified, non-ranked endpoint in the clinical trials.
I let my patients know that in the clinical trials, the average skin clearance on SKYRIZI was 58% from baseline at 4 weeks after just 1 dose, 91% clearance at 16 weeks after 2 doses, and 95% at 1 year after the 5th dose. In comparison, the PASI 90 response at Week 4 was 6%.
Individual results may vary for patients. 88% of PASI 90 responders at Week 16 maintained their response at Week 52.
Here, you can see rapid skin clearance on an actual patient from the UltlMMa-2 trial. As early as Week 4, this patient saw a 63% improvement in their skin after just 1 dose of SKYRIZI. At Week 16, the patient had 88% clearer skin, and at 1 year, the patient had 94% clearer skin from baseline.
Cynthia: In addition, SKYRIZI is dosed 4 times a year after 2 initiation doses—with 2 injections per dose. Patients have the flexibility to receive injections in-office or to self-inject after proper training.
Communicating the risks and benefits of a biologic like SKYRIZI is important. SKYRIZI has a well-studied safety profile across 4 pivotal trials, with a total of 1,306 patients receiving SKYRIZI. The adverse events through Week 16 included upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
With all biologics, I discuss important safety considerations. With SKYRIZI, I let patients know that warnings and precautions include risk of infections, tuberculosis, and avoiding the use of live vaccines.
There are no labeled warnings or precautions around malignancy, IBD, or depression. Also, there is no routine monitoring after an initial TB test.
Jason: In summary, I can communicate to my patients that SKYRIZI is proven to deliver high levels of skin clearance, including results as early as 4 weeks.
Cynthia: In addition, SKYRIZl's dosing schedule and well-studied safety profile are important pieces of my clear conversations with patients.
Indication
SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Safety Considerations
SKYRIZI may increase the risk of infection. Instruct patients to report signs or symptoms of clinically important infection during treatment. Should such an infection occur, discontinue SKYRIZI until infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with SKYRIZI. Avoid use of live vaccines in SKYRIZI patients.
Please see additional Important Safety Information at the bottom of this page. Please see full Prescribing Information by clicking the link at the top of this page.
US-SKZD-190188
If emailing to yourself, please provide only your own information.
US-SKZD-220459
SKYRIZI (risankizumab-rzaa) 150 mg/mL Pen Injection Video
INJECTING WITH THE SKYRIZI PEN
DISCLAIMER
Super: This demonstration is a guide to injecting with the SKYRIZI Pen. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject. Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
Audio: This demonstration is a guide to injecting with the SKYRIZI Pen. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject.
Super: SKYRIZI® (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
with active psoriatic arthritis (PsA).
Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
Audio: SKYRIZI (risankizumab-rzaa) uses
SKYRIZI is a prescription medicine used to treat adults: with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).with active psoriatic arthritis (PsA).
SUPER: SAFETY CONSIDERATIONS
SKYRIZI may cause serious side effects, including:
Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI.
Also, tell your doctor if you plan to or recently received a vaccine.
Please see Use and Important Safety Information at the end of this video. Please see link for the full Prescribing Information, including the medication guide, for SKYRIZI.
AUDIO: Safety considerations.
SKYRIZI may cause serious side effects, including:
Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious
allergic reaction.
Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. Also, tell your doctor if you plan to or recently received a vaccine.
Video: Cut to table shot with Sharps Container and Pen package.
SUPER: A GUIDE TO INJECTING WITH SKYRIZI
AUDIO: Hi. I’m Michael.
VIDEO: Cut to Michael facing camera.
SUPER: MICHAEL
AUDIO: And I’m here to show you how to inject using a SKYRIZI Pen.
Video: Cut to wider shot of Michael.
AUDIO: I’ve been doing this for awhile now, and I feel like I have a good handle on it. Ah, my doctor showed me how to inject when I first started.
VIDEO: Cut to phone with Nurse Ambassador screen displayed
SUPER: Don’t have a Nurse Ambassador?* Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
SKYRIZI logo
AUDIO: And I called Lauren, my Nurse Ambassador, and . . .
VIDEO: Michael sitting at table.
SUPER: Don’t have a Nurse Ambassador?* Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
AUDIO: . . . she helped me the first few times I did it at home by myself.
VIDEO: Cut to table shot of alcohol pad, Pen package, Sharps Container, and instructions.
SUPER: INJECTION PREP
AUDIO: Now, I get my SKYRIZI by mail . . .
VIDEO: Cut to Michael opening the refrigerator
AUDIO: . . . and I keep it in the fridge until I’m ready to inject.
Video: Shot continues, Michael reaches into refrigerator.
AUDIO: Ah, there it is.
Super: Shot continues, Michael closes refrigerator.
AUDIO: There we go.
VIDEO: Shot continues, Michael puts SKYRIZI package on table
SUPER: Do not warm SKYRIZI in any other way (for example, do not warm it in a microwave or in hot water).
AUDIO: So, very important.
Video: Shot continues as Michael continues talking.
Super: Do not use SKYRIZI if liquid has been frozen or even if it has been thawed.
I’ll leave it out at room temperature and out of direct sunlight for 30 to 90 minutes before injecting.
VIDEO: Shot continues, Michael walks off screen.
SUPER: 30 to 90 minutes
Keep SKYRIZI in the original carton to protect from light until time to use.
Do not use SKYRIZI if liquid has been frozen or even if it has been thawed.
VIDEO: Cut to Michael sitting at table
SUPER: Do not shake SKYRIZI.
AUDIO: Now that I’ve washed and dried my hands, I’m good to go.
Video: Shot continues, Michael continues talking.
SUPER: Do not use if the SKYRIZI Pen has been dropped or damaged.
AUDIO: So, I take out everything I need. Ah, I got my SKYRIZI Pen.
Video: Cut to table shot with phone, Pen, package, instructions, alcohol swab, Sharps Container, and cotton ball. Michael points at each
Super: SKYRIZI PEN
Do not use if the SKYRIZI Pen has been dropped or damaged.
Video: Shot continues, Michael continues talking and pointing at items.
Super: SKYRIZI PEN
INSTRUCTIONS FOR USE
ALCOHOL SWAB
not included in your package
Do not use SKYRIZI if package perforations are broken. Return product to the pharmacy.
Audio: I’ve got the Instructions for Use for reference. I’ve also got an alcohol swab . . .
Video: Shot continues, Michael continues talking and pointing at items
Super: SKYRIZI PEN
INSTRUCTIONS FOR USE
ALCOHOL SWAB
not included in your package
COTTON BALL
not included in your package
SHARPS CONTAINER
Do not remove the dark gray cap until right before injection.
Audio: . . . a cotton ball, and my sharps container. So . . .
Video: Cut to Michael sitting at table
Super: Do not remove the dark gray cap until right before injection.
Audio: . . . I do my injections . . .
Video: Cut to Michael sitting at table.
Super: Do not use SKYRIZI if expiration date (EXP) has passed.
Audio: . . . here because there’s just plenty of light and a lot of good space.
Video: Cut to table shot with alcohol swab, package with Pen, Sharps Container, and instructions.
Super: HOW TO INJECT
step-by-step
Audio: I like to think about the injection process like this . . .
Video: Cut to wide shot of inside of package.
Audio: Pick. Pull. Place. Push. And press. So first . . .
Video: Cut to wide shot of Michael indicating his thigh.
Super: PICK
the injection site
Audio: . . . I’ll pick my injection site. I’m gonna go with my left thigh. But you can also inject into your stomach or your other thigh.
Video: Shot continues, copy fades off screen, Michael indicates his stomach.
Audio: If you choose your stomach, make sure that you inject . . .
Video: Shot continues, Michael continues talking and indicating his stomach.
Super: 2 INCHES
Square graphic with arrows.
Audio: Grab your alcohol swab and clean your skin in . . .
Video: Shot continues, copy and graphic fade off screen, Michael continues talking.
Audio: Grab your alcohol swab and clean your skin in . . .
Video: Cut to close-up of Michael’s thigh. He rubs it with the alcohol swab.
Super: Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
Audio: . . . a circular motion like this. And let it dry.
Video: Cut to shot of Michael at table.
Super: Do not inject through clothes.
Audio: Now, I hold the Pen with the dark gray cap pointing up.
Video: Shot continues as Michael pulls the cap off of the Pen and continues talking.
Super: PULL
the cap off
Do not inject into skin that is sore, bruised, red, hard, scarred, has stretch marks, or areas with psoriasis.
Audio: I pull the cap straight off and throw it away. Now . . .
Video: Cut to close-up of Michael holding the pen.
Audio: . . . I check the liquid through the inspection window.
Video: Shot continues as Michael continues talking.
Super: Do not use if the liquid is cloudy or contains flakes or large particles.
Audio: It should look clear to slightly yellow and may contain tiny white or clear particles. It’s normal to see one or more bubbles in the liquid.
Video: Cut to wide shot of Michael holding Pen horizontally.
Audio: So, I hold my Pen with my fingers on the gray grips. See
Video: Shot continues as Michael turns the Pen and holds it vertically, indicating the activator button. Inset shot shows close up of needle sleeve.
Super: NEEDLE SLEEVE
Audio: And I turn the Pen so that the white needle sleeve points toward my injection site and I can see the green activator button.
Video: Cut to close-up of Pen showing injection window and needle sleeve.
Audio: I also make sure that I can clearly see the inspection window while I’m injecting.
Video: Cut to Michael lowering the Pen to his thigh and squeezing the skin at the injection site.
Audio: I gently squeeze my skin at my injection site and make a raised area and hold it firmly. Then . . .
Video: Shot continues as Michael continues talking and lowering the Pen to his thigh.
Super: PLACE
Audio: . . . I place the white needle sleeve straight . . .
Video: Cut to close-up of Michael holding the Pen against his thigh.
Super: PLACE & PUSH
against skin
90°
Right angle graphic
Audio: . . . against the raised area, at a 90 degree angle, and push the Pen down against my skin. Throughout the process, I keep pinching . . .
Video: Close-up of Michael injecting into his thigh.
Super: PLACE & PUSH
against skin
Audio: . . . the raised area and keep steady pressure against it
Video: Cut to different angle of Michael injecting.
Audio: Now I can see the green activator button and inspection window.
Video: Cut to split screen of Michael injecting.
Super: PUSH
against skin
The Pen will activate only if the white needle sleeve is firmly pushed down against the injection site before pressing the green activator button.
Audio: The Pen only activates if the white needle sleeve is pressed firmly down against me before pressing the green activator button. A loud . . .
Video: Cut to close up of Michael holding Pen against his thigh.
Super: PRESS
the green button and keep steady pressure
Audio: . . . click means the start of the injection.
Video: Clock graphic animates on and counts down from 00:15 seconds.
Super: PRESS
the green button and keep steady pressure
Audio: SFX: Click sound
There it goes. And hold it like that for 15 seconds.
Video: Shot continues as copy fades off screen.
Graphic on screen:
Clock graphic counting down
Audio: Now, I’ll keep pushing the Pen down against my skin until I hear a second click . . . or the yellow indicator has filled the inspection window—either one signals the injection is complete.
Video: Shot continues as graphics fade off screen and inset of injection window appears.
Audio: SFX: Click sound
And there it goes. I check to make sure that the yellow indicator is fully down to be sure the injection is complete. Only then do . . .
Video: Shot continues as Michael lifts the Pen from his thigh.
Audio: . . . I slowly pull the pen straight out from my skin.
SFX: Click sound
You might notice one last click.
Video: Cut to close-up of Michael indicating the white needle sleeve of the Pen.
Audio: And look at this. You see how the white needle sleeve covers the needle tip?
Video: Cut to wide shot of Michael holding the pen.
Audio: And that’s it.
Video: Shot continues as copy animates on screen.
Super: Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
Audio: Now, I press a cotton ball where I injected. You could also use a gauze pad.
Video: Shot continues as copy animates off screen and new copy fades in.
Super: Do not rub the injection site. You may have slight bleeding. This is normal.
Audio: And I drop my used Pen . . .
Video: Cut to close-up of Michael placing Pen in Sharps Container.
Super: Do not rub the injection site. You may have slight bleeding. This is normal.
Audio: . . . in one of these Sharps Disposal Containers.
Video: Michael carries the Sharps Container and places it on a high shelf.
Super: Your Skyrizi Complete Sharps Disposal Container may look a little different than the one shown.
Audio: There. Safe and sound and also out of reach.
Video: Cut to shot of Michael sitting at table.
Audio: Another injection complete.
Video: Cut to table shot with alcohol swab, package with Pen, Sharps Container, and instructions.
Super: HELPFUL TIPS
for self-injecting
Audio: To recap . . .
Video: Cut to wide shot of Michael sitting at table.
Audio: . . . I prepare myself and my space.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
Audio: Pick an injection site.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
PULL the cap off
Audio: Pull the cap off the Pen.
Video: Shot continues as screens with copy animate in
Super: PICK the injection sit
PULL the cap off
PLACE Pen on site
Audio: Place the Pen on the injection site.
Video: Shot continues as screens with copy animate in.
Super: PICK the injection site
PULL the cap off
PLACE Pen on site
PUSH firmly down against skin
Audio: Push the Pen firmly down against my skin.
Video: Shot continues as screens with copy animate in.
Super: PULL the cap off
PLACE Pen on site
PUSH firmly down against skin
PRESS the button
Audio: Press the button to inject. And keep steady pressure on the injection site for the full 15 seconds.
Video: Cut to close-up of Michael placing the lid on the Sharps Container.
Audio: Then safely discard the used Pen.
Video: Shot continues as Michael’s hands leave the frame.
Super: DISCARD used Pen
Audio: That’s the whole injection process.
Video: Cut to medium shot of Michael at table.
Audio: When I started injecting at home, it didn’t take me long to get it down. Lauren, my Nurse Ambassador, she suggested I create a routine. Inject at the same time and place.
Video: Cut to wide shot of Michael at table.
Audio: I also like to treat myself after each injection. And maybe I’ll cycle or listen to a new album.
Video: Cut to table shot. Michael indicates phone screen showing Skyrizi Complete App.
Audio: And this Skyrizi Complete App helps me stay on track, and I don’t know what I’d do without it.
Video: Cut to medium shot of Michael at table.
Audio: I used it to log my first starter dose, so I knew when to take my second starter dose four weeks later. And you can . . .
Video: Cut to table shot. Michael indicates phone screen showing a different screen of the Skyrizi Complete App.
Audio: . . . set reminders for future doses, too, since after two starter doses, . . .
Video: Cut to medium shot of Michael sitting at table.
Super: 1 INJECTION 4x A YEAR
Audio: . . . SKYRIZI is only 1 injection, 4 times a year.
Video: Cut to table shot showing phone with reminder message.
Audio: And when I get those reminders . . .
SFX: Ping sound
I know to contact my specialty pharmacy to get my next injection on time.
Video: Cut to wide shot of Michael at table.
Super: Get sharps containers at no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
Audio: And don’t forget about the Skyrizi Complete Sharps Disposal and mail-back service.
Video: Cut to table shot with alcohol swab and Michael putting lid on Sharps Container.
Super: Get sharps containers at no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
Audio: I signed up online, so once my Sharps Container gets full, . . .
Video: Cut to wide shot of Michael sitting at the table.
Audio: . . . I request a new one. Which is sent to me with a box to send my full one back. It’s so convenient, and it doesn’t cost me anything.
Video: Cut to medium shot of Michael sitting at the table.
Audio: And I’m lucky that my family’s always around if I need help.
Video: Shot continues, copy fades on screen.
Super: CALL 1.866.SKYRIZI
FOR 24/7 SUPPORT WITH
SKYRIZI COMPLETE
Audio: . . . and a pretty awesome app.
Video: Cut to wide shot of Michael sitting at table.
Audio: You’ve got this.
Video: Cut to USES and Important Safety Information screen. Copy on screen scrolls:
Super:
USES AND IMPORTANT SAFETY INFORMATION1
SKYRIZI® (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
with active psoriatic arthritis (PsA).
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SKYRIZI® (risankizumab-rzaa)?
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
USES AND IMPORTANT SAFETY INFORMATION
SKYRIZI (risankizumab-rzaa) USES
SKYRIZI is a prescription medicine used to treat adults:
with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
with active psoriatic arthritis (PsA).
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SKYRIZI (risankizumab-rzaa)?
Video: Scroll continues.
Super: SKYRIZI is a prescription medicine that may cause serious side effects, including:
Serious allergic reactions:
Stop using SKYRIZI and get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
SKYRIZI is a prescription medicine that may cause serious side effects, including:
Serious allergic reactions:
Stop using SKYRIZI and get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
Video: Scroll continues.
Super:
Infections:
SKYRIZI may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with SKYRIZI and may treat you for TB before you begin treatment with SKYRIZI if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with SKYRIZI.
Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
Infections
SKYRIZI may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with SKYRIZI and may treat you for TB before you begin treatment with SKYRIZI if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with SKYRIZI.
Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
Video: Scroll continues.
Super:
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
Before using SKYRIZI, tell your healthcare provider about all of your medical conditions, including if you:
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
Before using SKYRIZI, tell your healthcare provider about all of your medical conditions, including if you:
Video: Scroll continues.
Super:
have any of the conditions or symptoms listed in the section “What is the most important information I should know about SKYRIZI?”
have an infection that does not go away or that keeps coming back.
have TB or have been in close contact with someone with TB.
have recently received or are scheduled to receive an immunization (vaccine). Medications that interact with the immune system may increase your risk of getting an infection after receiving live vaccines. You should avoid receiving live vaccines right before, during, or right after treatment with SKYRIZI. Tell your healthcare provider that you are taking SKYRIZI before receiving a vaccine.
are pregnant or plan to become pregnant. It is not known if SKYRIZI can harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if SKYRIZI passes into your breast milk.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
have any of the conditions or symptoms listed in the section “What is the most important information I should know about SKYRIZI?”
have an infection that does not go away or that keeps coming back.
have TB or have been in close contact with someone with TB.
have recently received or are scheduled to receive an immunization (vaccine). Medications that interact with the immune system may increase your risk of getting an infection after receiving live vaccines. You should avoid receiving live vaccines right before, during, or right after treatment with SKYRIZI. Tell your healthcare provider that you are taking SKYRIZI before receiving a vaccine.
are pregnant or plan to become pregnant. It is not known if SKYRIZI can harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if SKYRIZI passes into your breast milk.
Video: Scroll continues.
Super:
become pregnant while taking SKYRIZI. You are encouraged to enroll in the Pregnancy Registry, which is used to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-302-2161 to enroll in this registry.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of SKYRIZI?
SKYRIZI may cause serious side effects. See “What is the most important information I should know about SKYRIZI?”
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
become pregnant while taking SKYRIZI. You are encouraged to enroll in the Pregnancy Registry, which is used to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-302-2161 to enroll in this registry.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of SKYRIZI?
SKYRIZI may cause serious side effects. See “What is the most important information I should know about SKYRIZI?”
Video: Scroll continues.
Super:
The most common side effects of SKYRIZI in people treated for plaque psoriasis and psoriatic arthritis include: upper respiratory infections, headache, feeling tired, injection site reactions, and fungal skin infections.
These are not all the possible side effects of SKYRIZI. Call your doctor for medical advice about side effects.
Use SKYRIZI exactly as your healthcare provider tells you to use it.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI
SKYRIZI logo.
Audio:
The most common side effects of SKYRIZI in people treated for plaque psoriasis and psoriatic arthritis include: upper respiratory infections, headache, feeling tired, injection site reactions, and fungal skin infections.
These are not all the possible side effects of SKYRIZI. Call your doctor for medical advice about side effects.
Use SKYRIZI exactly as your healthcare provider tells you to use it.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Video: Scroll continues.
Super:
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/myAbbVieAssist to learn more.
Reference: 1. SKYRIZI [package insert]. North Chicago, IL. AbbVie Inc.
US-SKZD-220756
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI.
SKYRIZI logo
Audio:
If you are having difficulty paying for your medicine, AbbVie may be able to help.
Visit AbbVie.com/myAbbVieAssist to learn more.
Please see link for the full Prescribing Information, including the Medication Guide, for SKYRIZI
US-SKZD-230756
SKYRIZI (risankizumab-rzaa) 150 mg/mL Prefilled Syringe Injection Video
SYRINGE INJECTION VIDEO
DISCLAIMER
SUPER: This demonstration is a guide to injecting with the SKYRIZI prefilled syringe. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject. Please see Instructions for Use and Important Safety Information within the website. Please see accompanying full Prescribing Information, including Medication Guide, and discuss with your doctor.
AUDIO: This demonstration is a guide to injecting with the SKYRIZI prefilled syringe. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject
VIDEO: A GUIDE TO INJECTING SKYRIZI
VIDEO: We see the patient comfortably seated on a couch in their living room. Patient looks into camera and introduces himself.
SUPER: MAYA
AUDIO: Hey there! I’m Maya and I’m here to walk you through how to inject yourself with SKYRIZI. Now, if it’s your first time, don’t worry! If it’s not your first time, I hope you pick up some new tips while you’re here.
VIDEO: Patient speaks to camera, points to “Bryan (Nurse Ambassador)” contact on phone.
SUPER:
Don’t have a Nurse Ambassador?*
Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
AUDIO: Now, I’ve done this a few times by myself, but my doctor originally walked me through how to inject, and I called Bryan, who’s my Nurse Ambassador, when I first started injecting on my own at home.
VIDEO: Patient stands and walks out of frame. INJECTION PREP
VIDEO: Patient walks to fridge, pulls out SKYRIZI package, shows to camera, places on counter. SUPERS rotate through on screen.
SUPER: Do not warm SKYRIZI in any other way (for example, do not warm it in a microwave or in hot water).
Do not use SKYRIZI if liquid has been frozen even if it has been thawed.
15 to 30 minutes
Keep SKYRIZI in the original carton to protect from light until time to use.
AUDIO: Alright, let’s get started. So, when my SKYRIZI comes in the mail, it goes straight in the fridge. Now, before I inject, I let it sit out for 15 to 30 minutes, out of direct sunlight, to get to room temperature. And while I wait, I try to relax.
VIDEO: Patient sits down on couch with items needed on the table in front of her, SUPERS rotate through on screen. Supply names animate on screen next to each item, as she speaks their names.
SUPER:
Do not shake SKYRIZI.
Do not use SKYRIZI if the syringe has been dropped or damaged.
Do not use SKYRIZI if package perforations are broken. Return product to the pharmacy.
Do not remove the needle cover until right before injection.
Do not use SKYRIZI if expiration date (EXP) has passed.
SKYRIZI syringe, with cardboard sleeve removed, alcohol swab not included in your package, Instructions for Use, cotton ball not included in your package, sharps container
AUDIO: Okay, that should be enough time. So, once my hands are clean, I like to put out everything that I’ll need. So, I have my SKYRIZI syringe. I have an alcohol swab, which I got from my local pharmacy. I also have the Instructions for Use, just in case. And I also like to grab a cotton ball (I had some right in my medicine cabinet) and then a sharps container. Now, I like this spot because there’s a lot of space and I don’t have to leave my couch.
VIDEO: HOW TO INJECT step-by-step
VIDEO: Patient points to 4 Ps graphic on package.
AUDIO: So, it helps me to break down the injection process into 4 simple steps: the 4 Ps. And you can see them here on the package. We have: pick, prepare, pinch, push. Okay?
SUPER: PICK the injection site
AUDIO: So, first, I pick my injection site. So, I’ll go with my left thigh. But, you can also pick your right thigh or your lower stomach.
ANIMATION: 2 inches graphic animates.
SUPER: 2 INCHES
AUDIO: But, if you choose your stomach, make sure to inject at least 2 inches away from your belly button. Okay?
VIDEO: Patient demonstrates step.
AUDIO: So, you want to grab your alcohol swab to clean your skin in a circular motion, like this okay, and let it dry.
VIDEO: Patient holds syringe with needle pointing down, removes cap to check liquid, shows to camera. SUPERS rotate through on screen.
SUPER:
Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
Do not inject through clothes.
Do not inject into skin that is sore, bruised, red, hard, scarred, or has stretch marks.
Do not use if the liquid is cloudy or contains flakes or large particles.
AUDIO: Now, I hold my syringe with the needle facing down, and I check the liquid inside to make sure that everything looks okay. It should be clear to slightly yellow, which it is. See that? There are a few bubbles in there, but that’s normal. Looks like we’re good to go!
VIDEO: Patient removes the needle cover by holding the syringe in one hand and gently pulling the needle cover straight off with the other hand.
SUPER:
PREPARE the syringe
Do not hold or pull plunger when removing the needle cover.
Do not touch the needle with your fingers or let the needle touch anything.
AUDIO: Alright, so I hold my syringe in one hand, and I take the needle cover off with the other hand, okay? Like this, and then you want to throw it away. You see? You may see a drop of liquid at the end of the needle, which is normal, okay?
VIDEO: Patient demonstrates steps.
SUPER:
PINCH the skin
AUDIO: So, I’ve got my syringe in one hand, and with the other hand, I’m gently pinching the area of my cleaned skin to hold firmly.
VIDEO: Patient demonstrates step.
SUPER:
PUSH the plunger in
45°
AUDIO: Now I’ll inject the needle into my skin at about a 45-degree angle using a quick, short movement — like this. Now, holding the angle steady, I slowly push the plunger in all the way until all of the liquid is injected, and the syringe is empty. There we go.
VIDEO: Patient demonstrates step.
AUDIO: Now, I pull the needle out of my skin while keeping the syringe at the same angle, like this. I release the plunger to allow the syringe to move up.
VIDEO: Patient points to needle guard on syringe.
AUDIO: Until the entire needle is covered up by the needle guard. See how that works? The needle guard won’t come back down unless all the liquid has been injected.
VIDEO: Patient demonstrates step. SUPERS rotate through on screen.
SUPER:
Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
Do not rub the injection site. You may have slight bleeding. This is normal.
AUDIO: Alright, so now, I press my cotton ball where I just injected, and that’s it!
VIDEO: Patient demonstrates step.
SUPER:
Do not rub the injection site. You may have slight bleeding. This is normal.
AUDIO: I drop my used syringe in one of these handy sharps disposal containers. Voilá – safe and sound.
VIDEO: Patient places the sharps container on closet shelf.
AUDIO: Now I’ll just put it back out of reach. Just like that, another injection complete.
VIDEO: HELPFUL TIPS for self-injecting
ANIMATION/SUPER:
PICK the injection site
PREPARE the syringe
PINCH the skin
PUSH the plunger in
Text animates on screen for each step.
AUDIO: So, to recap, I prepare myself and my space. I pick an injection site, prepare the syringe, pinch the skin, push the plunger in.
VIDEO: Patient demonstrates step.
AUDIO: And safely discard the syringe. That’s the whole injection process!
VIDEO: Patient speaks to camera.
AUDIO: Now, it seemed tricky at first, but I got it down soon enough. And Bryan, my Nurse Ambassador, he recommended I create a routine — injecting at the same time, same place — that sort of thing. I also do something nice for myself after each injection — maybe I’ll paint as a reward.
VIDEO: Patient holds up phone to camera.
AUDIO: Oh, and this Skyrizi Complete App has helped me so much.
VIDEO: Patient points to App on phone.
AUDIO: I mean I used it to log my first starter dose so I knew when to take my second starter dose 4 weeks later. You can set reminders for future doses, too.
VIDEO: Patient speaks to camera.
SUPER: 1 INJECTION 4x A YEAR
AUDIO: Since, after 2 starter doses, SKYRIZI is only 1 injection 4 times a year.
VIDEO: Patient points to phone.
App notification reminder appears on phone screen.
AUDIO: And when I get those reminders, I know to contact my specialty pharmacy to get my next injection on time.
VIDEO: Patient speaks to camera.
SUPER: Get sharps containers for no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
AUDIO: Oh, and I can’t forget about the Skyrizi Complete sharps disposal and mail-back service.
ANIMATION: SUPER animates over sharps container
SUPER: Get sharps containers for no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
FULL
AUDIO: Once my sharps container gets full, I request a new one that’s sent to me with a box to mail this one back. I signed up for the service online, and it doesn’t cost me anything!
VIDEO: Patient speaks to camera. SUPER animates on screen.
SUPER: CALL 1.866.SKYRIZI FOR 24/7 SUPPORT WITH SKYRIZI COMPLETE
AUDIO: I’m lucky to have people around to support me. I’ve got Skyrizi Complete on my team, too. Like Bryan, who’s such a good listener,
VIDEO: Patient picks up phone from table.
SUPER: CALL 1.866.SKYRIZI FOR 24/7 SUPPORT WITH SKYRIZI COMPLETE
AUDIO: and this App!
VIDEO: Patient speaks to camera.
AUDIO: There’s always someone to reach out to if you need help. You’ve got this.
VIDEO: SAFETY CONSIDERATIONS1
SKYRIZI may cause serious side effects, including:
Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI.
Also, tell your doctor if you plan to or recently received a vaccine.
REFERENCE: 1. SKYRIZI [package insert]. North Chicago, IL: AbbVie, Inc.
US-SKZD-220457
Plaque Psoriasis: SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Psoriatic Arthritis: SKYRIZI is indicated for the treatment of active psoriatic arthritis in adults.
Hypersensitivity Reactions
SKYRIZI® (risankizumab-rzaa) is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab-rzaa or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of SKYRIZI. If a serious hypersensitivity reaction occurs, discontinue SKYRIZI and initiate appropriate therapy immediately.
Infection
SKYRIZI may increase the risk of infection. Do not initiate treatment with SKYRIZI in patients with a clinically important active infection until it resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing SKYRIZI. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, closely monitor and discontinue SKYRIZI until the infection resolves.
Tuberculosis (TB)
Prior to initiating treatment with SKYRIZI, evaluate for TB infection and consider treatment in patients with latent or active TB for whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SKYRIZI treatment. Do not administer SKYRIZI to patients with active TB.
Administration of Vaccines
Avoid use of live vaccines in patients treated with SKYRIZI. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating SKYRIZI, complete all age appropriate vaccinations according to current immunization guidelines.
Adverse Reactions
Most common (≥1%) adverse reactions associated with SKYRIZI include upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
In psoriatic arthritis phase 3 trials, the incidence of hepatic events was higher with SKYRIZI compared to placebo.
SKYRIZI is available in a 150 mg/mL prefilled syringe and pen.
Please see Full Prescribing Information.
US-SKZD-210594



