The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active Crohn's disease (CD) in adults.1
SKYRIZI vs STELARA® (ustekinumab)
SKYRIZI ACHIEVED SUPERIORITY ACROSS SEVERAL RANKED ENDPOINTS IN A HEAD-TO-HEAD STUDY
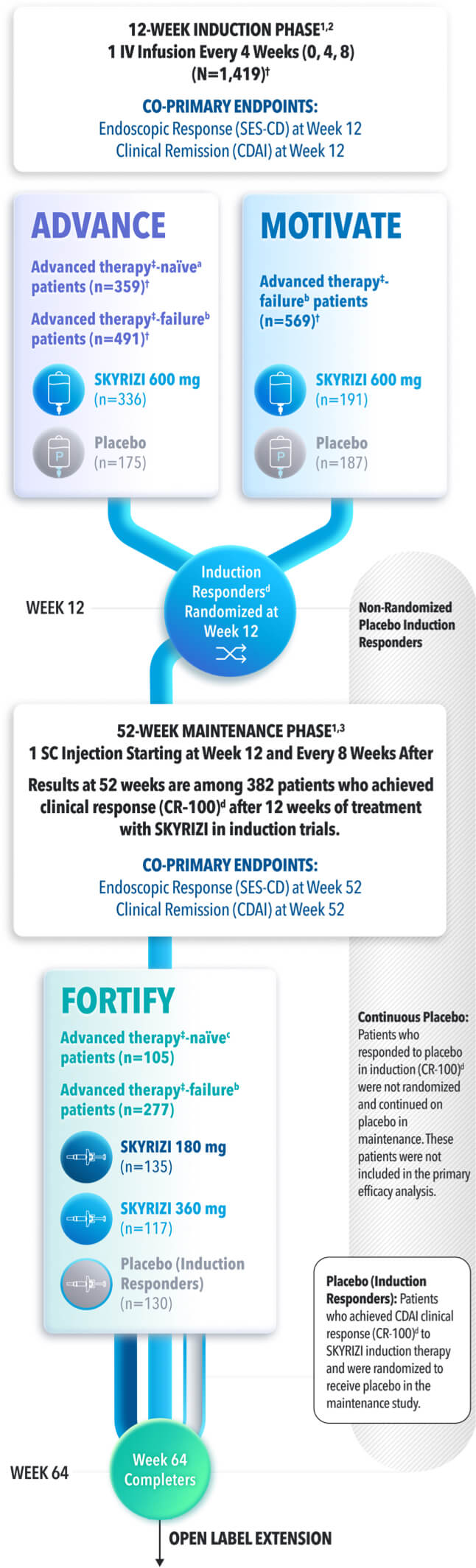
ENDPOINTS IN ADVANCE, MOTIVATE AND FORTIFY1

All p-values are SKYRIZI treatment arms vs. placebo.
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR-100)‡ to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.
STUDY DESIGNS:
ADVANCE (N=850) and MOTIVATE (N=569) Induction studies were 12-week, randomized, double-blind, placebo-controlled studies that evaluated the efficacy and safety of SKYRIZI in patients with moderately to severely active Crohn’s disease§ who demonstrated prior treatment failure to conventional and/or biologic treatment.3 Patients received an IV infusion of SKYRIZI 600 mg (recommended dose), risankizumab-rzaa 1200 mg|| or placebo at Weeks 0, 4, and 8.1
FORTIFY (N=382) Maintenance study was a 52-week study that evaluated the efficacy and safety of SKYRIZI in patients who achieved clinical response (decrease in CDAI ≥100)‡ from SKYRIZI induction in the ADVANCE and MOTIVATE studies. Patients were randomized to SKYRIZI 180 mg SC, SKYRIZI 360 mg SC, or placebo at Week 12 and every 8 weeks thereafter.1
First Approved Crohn's Disease Therapy
That Met Endoscopic Superiority Endpoints
in a
Head-to-Head Trial2,3

PRIMARY ENDPOINTS EVALUATED:
- Non-Inferiority Endpoint: CLINICAL REMISSION (CDAI <150) AT WEEK 24*, Endpoint Met
- Superiority Endpoint: ENDOSCOPIC REMISSION† (SES-CD ≤4) AT WEEK 48‡, Endpoint Met
*The percentage of subjects who met clinical remission at Week 24. Statistical requirement for non-inferiority achieved with ~50% of study population.
†Endoscopic remission: SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a
central reviewer.
‡The percentage of subjects who met endoscopic remission at Week 48.
Study Design Intro: SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded§ study of SKYRIZI (n=255) compared to STELARA (ustekinumab)|| (n=265) for the treatment of adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy. Eligible patients were randomized (1:1) to receive either SKYRIZI (600 mg IV to 360 mg SC) or STELARA (weight-based¶ IV to 90 mg SC). After induction dosing was completed, patients remained on their respective therapy throughout the duration of the maintenance period (treat-through study design). No dose escalation allowed throughout the trial.
Dosing: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
§The investigator and site personnel were blinded to the results of the clinical outcomes (CDAI) and endoscopies were centrally read with assessors blinded
to study drug.
||Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
¶Baseline Stelara IV dose is weight based: ≤55 kg: 260 mg; >55 kg to 85 kg: 390 mg; or >85 kg: 520 mg.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
HEAD-TO-HEAD TRIAL: PRIMARY ENDPOINTS (NRI-MI)2,3
SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded* head-to-head study comparing
SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.

~2X MORE PATIENTS on SKYRIZI Demonstrated Endoscopic Remission vs STELARA at Week 48
Patients at baseline had an average disease duration of ~9 years and average SES-CD
score of 14

SKYRIZI DEMONSTRATED SYMPTOM RELIEF AS MEASURED BY CLINICAL REMISSION AT WEEK 24
~2X MORE PATIENTS on SKYRIZI Demonstrated Endoscopic Remission vs STELARA at Week 48
Patients at baseline had an average disease duration of ~9 years and average SES-CD
score of 14

SKYRIZI DEMONSTRATED SYMPTOM RELIEF AS MEASURED BY CLINICAL REMISSION AT WEEK 24
Data Limitation: The open-label nature of this study may have influenced these results.
Dosing: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
*The investigator and site personnel were blinded to the results of the clinical outcomes (CDAI) and endoscopies were centrally read with assessors blinded to study drug.
†Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
SUPERIORITY MET ACROSS ENDOSCOPIC OUTCOMES2,3
Endoscopic Response at Week 24 and Week 48 (Ranked Secondary Endpoints, NRI-MI)
SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded* head-to-head study comparing
SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.

DATA LIMITATION: The open-label nature of this study may have influenced these results.
DOSING: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
*Endoscopies were centrally read with assessors blinded to study drug.
†Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
SUPERIORITY MET ACROSS ENDOSCOPIC OUTCOMES2,3
Endoscopic Remission at Week 48 (Primary Endpoint, NRI-MI)
SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded* head-to-head study comparing
SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.

~2x More Patients on SKYRIZI Demonstrated Endoscopic Remission vs Stelara at Week 48
PATIENTS AT BASELINE HAD AN AVERAGE DISEASE DURATION OF ~9 YEARS AND AVERAGE SES-CD SCORE OF 14
~2x More Patients on SKYRIZI Demonstrated Endoscopic Remission vs Stelara at Week 48
PATIENTS AT BASELINE HAD AN AVERAGE DISEASE DURATION OF ~9 YEARS AND AVERAGE SES-CD SCORE OF 14
Data Limitation: The open-label nature of this study may have influenced these results.
Dosing: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
*Endoscopies were centrally read with assessors blinded to study drug.
†Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
SUPERIORITY MET ACROSS ENDOSCOPIC OUTCOMES2,3
Steroid-Free Endoscopic Remission at Week 48 (Ranked Secondary Endpoint, NRI-MI)
SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded* head-to-head study comparing
SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.

~2x More Patients on SKYRIZI were in Endoscopic Remission without Steroids (Steroid-Free Endoscopic Remission) vs STELARA at Week 48
IN THE SEQUENCE TRIAL, 71 (27%) AND 58 (23%) PATIENTS WERE TAKING CORTICOSTEROID AT BASELINE IN THE STELARA AND SKYRIZI ARMS, RESPECTIVELY.
~2x More Patients on SKYRIZI were in Endoscopic Remission without Steroids (Steroid-Free Endoscopic Remission) vs STELARA at Week 48
IN THE SEQUENCE TRIAL, 71 (27%) AND 58 (23%) PATIENTS WERE TAKING CORTICOSTEROID AT BASELINE IN THE STELARA AND SKYRIZI ARMS, RESPECTIVELY.
Data Limitation: The open-label nature of this study may have influenced these results.
Dosing: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
Steroid-free clinical remission in the FORTIFY trial was not statistically significant under the pre-specified multiple testing procedure.
Steroid-free Endoscopic Remission: Total population of patients who achieved endoscopic remission, defined as SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer, who also did not receive a steroid at Week 48.
*Endoscopies were centrally read with assessors blinded to study drug.
†Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
DEMONSTRATED SYMPTOM RELIEF DATA2,3
Clinical Remission at Week 24 and Week 48 (NRI-MI)
SEQUENCE was a Phase 3, multicenter, randomized, open-label, efficacy assessment-blinded* head-to-head study comparing
SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.

SKYRIZI DEMONSTRATED SYMPTOM RELIEF AS MEASURED BY CLINICAL REMISSION AT WEEK 24 AND WEEK 48
SKYRIZI DEMONSTRATED SYMPTOM RELIEF AS MEASURED BY CLINICAL REMISSION AT WEEK 24 AND WEEK 48
Data Limitation: The open-label nature of this study may have influenced these results.
Dosing: The lowest effective dosage for SKYRIZI should be used to maintain therapeutic response. The comparative effectiveness of SKYRIZI 180 mg is unknown, as it was not evaluated in this study.
*The investigator and site personnel were blinded to the results of the clinical outcomes (CDAI).
†Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
SAFETY PROFILE: EVALUATION THROUGH WEEK 482,3
SEQUENCE was a Phase 3, multicenter, randomized, open-label, study of SKYRIZI and STELARA (ustekinumab) in adult patients with moderate to severe Crohn's disease who have failed anti-TNF therapy.
For the safety population, 7 patients randomized to SKYRIZI received 1200 mg IV and/or 180 mg SC and were included only in the safety analysis.
Safety Data presented includes all patients who received at least 1 dose of study drug.
*Active Comparator: 31 patients received US approved ustekinumab. All other patients received European Union approved ustekinumab. The comparability between US and Non US approved ustekinumab has not been established.
®STELARA is a registered trademark of Johnson & Johnson. See US Prescribing Information for further information.
The safety profile of SKYRIZI was generally consistent
with previously reported studies and as described in the
full Prescribing Information.
The Safety Rates observed in clinical trials may not reflect
clinical practice.